+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human UCP1 in the DNP-bound state | ||||||||||||||||||
 Map data Map data | Sharpened map | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | UCP1 / SLC25A7 / thermogenin / SLC25 / MEMBRANE PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpurine ribonucleotide binding / cellular response to dehydroepiandrosterone / Mitochondrial Uncoupling / The fatty acid cycling model / oxidative phosphorylation uncoupler activity / mitochondrial transmembrane transport / adaptive thermogenesis / cardiolipin binding / regulation of reactive oxygen species biosynthetic process / cellular response to cold ...purine ribonucleotide binding / cellular response to dehydroepiandrosterone / Mitochondrial Uncoupling / The fatty acid cycling model / oxidative phosphorylation uncoupler activity / mitochondrial transmembrane transport / adaptive thermogenesis / cardiolipin binding / regulation of reactive oxygen species biosynthetic process / cellular response to cold / cellular response to fatty acid / response to temperature stimulus / long-chain fatty acid binding / diet induced thermogenesis / proton transmembrane transporter activity / transmembrane transporter activity / brown fat cell differentiation / Transcriptional regulation of brown and beige adipocyte differentiation by EBF2 / cellular response to hormone stimulus / response to cold / proton transmembrane transport / response to nutrient levels / cellular response to reactive oxygen species / GDP binding / positive regulation of cold-induced thermogenesis / mitochondrial inner membrane / GTP binding / regulation of transcription by RNA polymerase II / mitochondrion Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.51 Å | ||||||||||||||||||
 Authors Authors | Chen L / Kang Y | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural basis for the binding of DNP and purine nucleotides onto UCP1. Authors: Yunlu Kang / Lei Chen /  Abstract: Uncoupling protein 1 (UCP1) conducts protons through the inner mitochondrial membrane to uncouple mitochondrial respiration from ATP production, thereby converting the electrochemical gradient of ...Uncoupling protein 1 (UCP1) conducts protons through the inner mitochondrial membrane to uncouple mitochondrial respiration from ATP production, thereby converting the electrochemical gradient of protons into heat. The activity of UCP1 is activated by endogenous fatty acids and synthetic small molecules, such as 2,4-dinitrophenol (DNP), and is inhibited by purine nucleotides, such as ATP. However, the mechanism by which UCP1 binds to these ligands remains unknown. Here we present the structures of human UCP1 in the nucleotide-free state, the DNP-bound state and the ATP-bound state. The structures show that the central cavity of UCP1 is open to the cytosolic side. DNP binds inside the cavity, making contact with transmembrane helix 2 (TM2) and TM6. ATP binds in the same cavity and induces conformational changes in TM2, together with the inward bending of TM1, TM4, TM5 and TM6 of UCP1, resulting in a more compact structure of UCP1. The binding site of ATP overlaps with that of DNP, suggesting that ATP competitively blocks the functional engagement of DNP, resulting in the inhibition of the proton-conducting activity of UCP1. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35928.map.gz emd_35928.map.gz | 77.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35928-v30.xml emd-35928-v30.xml emd-35928.xml emd-35928.xml | 15.9 KB 15.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35928.png emd_35928.png | 49.2 KB | ||
| Others |  emd_35928_half_map_1.map.gz emd_35928_half_map_1.map.gz emd_35928_half_map_2.map.gz emd_35928_half_map_2.map.gz | 77.7 MB 77.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35928 http://ftp.pdbj.org/pub/emdb/structures/EMD-35928 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35928 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35928 | HTTPS FTP |
-Validation report
| Summary document |  emd_35928_validation.pdf.gz emd_35928_validation.pdf.gz | 787.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35928_full_validation.pdf.gz emd_35928_full_validation.pdf.gz | 787 KB | Display | |
| Data in XML |  emd_35928_validation.xml.gz emd_35928_validation.xml.gz | 12.7 KB | Display | |
| Data in CIF |  emd_35928_validation.cif.gz emd_35928_validation.cif.gz | 14.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35928 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35928 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35928 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35928 | HTTPS FTP |
-Related structure data
| Related structure data |  8j1nMC  8hbvC  8hbwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_35928.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35928.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.834 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map A
| File | emd_35928_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_35928_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : UCP1-sybody complex
| Entire | Name: UCP1-sybody complex |
|---|---|
| Components |
|
-Supramolecule #1: UCP1-sybody complex
| Supramolecule | Name: UCP1-sybody complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Mitochondrial brown fat uncoupling protein 1
| Macromolecule | Name: Mitochondrial brown fat uncoupling protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.468367 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSRLEEELRR RLTEGSGGWS HPQFEKGSGD YKDDDDKGSG WSHPQFEKLE VLFQGPMGGL TASDVHPTLG VQLFSAGIAA CLADVITFP LDTAKVRLQV QGECPTSSVI RYKGVLGTIT AVVKTEGRMK LYSGLPAGLQ RQISSASLRI GLYDTVQEFL T AGKETAPS ...String: MSRLEEELRR RLTEGSGGWS HPQFEKGSGD YKDDDDKGSG WSHPQFEKLE VLFQGPMGGL TASDVHPTLG VQLFSAGIAA CLADVITFP LDTAKVRLQV QGECPTSSVI RYKGVLGTIT AVVKTEGRMK LYSGLPAGLQ RQISSASLRI GLYDTVQEFL T AGKETAPS LGSKILAGLT TGGVAVFIGQ PTEVVKVRLQ AQSHLHGIKP RYTGTYNAYR IIATTEGLTG LWKGTTPNLM RS VIINCTE LVTYDLMKEA FVKNNILADD VPCHLVSALI AGFCATAMSS PVDVVKTRFI NSPPGQYKSV PNCAMKVFTN EGP TAFFKG LVPSFLRLGS WNVIMFVCFE QLKRELSKSR QTMDCAT UniProtKB: Mitochondrial brown fat uncoupling protein 1 |
-Macromolecule #2: Sybody 12F2
| Macromolecule | Name: Sybody 12F2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 15.004661 KDa |
| Sequence | String: MGQVQLVESG GGLVQAGGSL RLSCAASGFP VMYYNMHWYR QAPGKEREWV AAIESTGWWA HYADSVKGRF TISRDNAKNT VYLQMNSLK PEDTAVYYCN VKDFGWRWEA YDYWGQGTQV TVSSLEHHHH HH |
-Macromolecule #3: 2,4-DINITROPHENOL
| Macromolecule | Name: 2,4-DINITROPHENOL / type: ligand / ID: 3 / Number of copies: 1 / Formula: DNF |
|---|---|
| Molecular weight | Theoretical: 184.106 Da |
| Chemical component information | 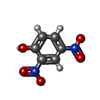 ChemComp-DNF: |
-Macromolecule #4: CARDIOLIPIN
| Macromolecule | Name: CARDIOLIPIN / type: ligand / ID: 4 / Number of copies: 1 / Formula: CDL |
|---|---|
| Molecular weight | Theoretical: 1.464043 KDa |
| Chemical component information |  ChemComp-CDL: |
-Macromolecule #5: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 5 / Number of copies: 2 / Formula: PC1 |
|---|---|
| Molecular weight | Theoretical: 790.145 Da |
| Chemical component information |  ChemComp-PC1: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.51 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 709625 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)