[English] 日本語
 Yorodumi
Yorodumi- EMDB-35049: Cryo-EM structure of beta-estradiol 17-(beta-D-glucuronide)-bound... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of beta-estradiol 17-(beta-D-glucuronide)-bound human ABC transporter ABCC3 in nanodiscs | |||||||||
 Map data Map data | beta-estradiol 17-(beta-D-glucuronide)- bound ABCC3 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / TRANSPORT PROTEIN / multidrug resistance protein / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationglucuronoside transmembrane transporter activity / icosanoid transmembrane transporter activity / ABC-type bile acid transporter activity / leukotriene transport / ABC-type glutathione-S-conjugate transporter / ABC-type glutathione S-conjugate transporter activity / ATPase-coupled inorganic anion transmembrane transporter activity / xenobiotic transmembrane transport / ABC-type xenobiotic transporter / Paracetamol ADME ...glucuronoside transmembrane transporter activity / icosanoid transmembrane transporter activity / ABC-type bile acid transporter activity / leukotriene transport / ABC-type glutathione-S-conjugate transporter / ABC-type glutathione S-conjugate transporter activity / ATPase-coupled inorganic anion transmembrane transporter activity / xenobiotic transmembrane transport / ABC-type xenobiotic transporter / Paracetamol ADME / NFE2L2 regulating MDR associated enzymes / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / ABC-type xenobiotic transporter activity / bile acid and bile salt transport / xenobiotic transport / Aspirin ADME / xenobiotic transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / transport across blood-brain barrier / ABC-type transporter activity / Recycling of bile acids and salts / xenobiotic metabolic process / basal plasma membrane / ABC-family proteins mediated transport / transmembrane transport / basolateral plasma membrane / ATP hydrolysis activity / ATP binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.65 Å | |||||||||
 Authors Authors | Wang J / Wang FF / Chen YX / Zhou CZ | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2023 Journal: EMBO J / Year: 2023Title: Placing steroid hormones within the human ABCC3 transporter reveals a compatible amphiphilic substrate-binding pocket. Authors: Jie Wang / Xu Li / Fang-Fang Wang / Meng-Ting Cheng / Yao-Xu Mao / Shu-Cheng Fang / Liang Wang / Cong-Zhao Zhou / Wen-Tao Hou / Yuxing Chen /  Abstract: The human ABC transporter ABCC3 (also known as MRP3) transports a wide spectrum of substrates, including endogenous metabolites and exogenous drugs. Accordingly, it participates in multiple ...The human ABC transporter ABCC3 (also known as MRP3) transports a wide spectrum of substrates, including endogenous metabolites and exogenous drugs. Accordingly, it participates in multiple physiological processes and is involved in diverse human diseases such as intrahepatic cholestasis of pregnancy, which is caused by the intracellular accumulation of bile acids and estrogens. Here, we report three cryogenic electron microscopy structures of ABCC3: in the apo-form and in complexed forms bound to either the conjugated sex hormones β-estradiol 17-(β-D-glucuronide) and dehydroepiandrosterone sulfate. For both hormones, the steroid nuclei that superimpose against each other occupy the hydrophobic center of the transport cavity, whereas the two conjugation groups are separated and fixed by the hydrophilic patches in two transmembrane domains. Structural analysis combined with site-directed mutagenesis and ATPase activity assays revealed that ABCC3 possesses an amphiphilic substrate-binding pocket able to hold either conjugated hormone in an asymmetric pattern. These data build on consensus features of the substrate-binding pocket of MRPs and provide a structural platform for the rational design of inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35049.map.gz emd_35049.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35049-v30.xml emd-35049-v30.xml emd-35049.xml emd-35049.xml | 15.9 KB 15.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35049.png emd_35049.png | 103.8 KB | ||
| Filedesc metadata |  emd-35049.cif.gz emd-35049.cif.gz | 6.4 KB | ||
| Others |  emd_35049_half_map_1.map.gz emd_35049_half_map_1.map.gz emd_35049_half_map_2.map.gz emd_35049_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35049 http://ftp.pdbj.org/pub/emdb/structures/EMD-35049 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35049 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35049 | HTTPS FTP |
-Validation report
| Summary document |  emd_35049_validation.pdf.gz emd_35049_validation.pdf.gz | 839.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_35049_full_validation.pdf.gz emd_35049_full_validation.pdf.gz | 839.4 KB | Display | |
| Data in XML |  emd_35049_validation.xml.gz emd_35049_validation.xml.gz | 12.1 KB | Display | |
| Data in CIF |  emd_35049_validation.cif.gz emd_35049_validation.cif.gz | 14.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35049 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35049 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35049 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-35049 | HTTPS FTP |
-Related structure data
| Related structure data |  8hw2MC  8hvhC  8hw4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35049.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35049.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | beta-estradiol 17-(beta-D-glucuronide)- bound ABCC3 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
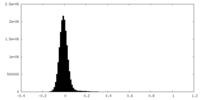
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: E217 halfB
| File | emd_35049_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E217_halfB | ||||||||||||
| Projections & Slices |
| ||||||||||||
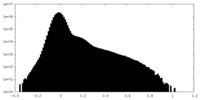
| Density Histograms |
-Half map: E217 halfB
| File | emd_35049_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E217_halfB | ||||||||||||
| Projections & Slices |
| ||||||||||||
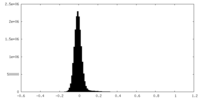
| Density Histograms |
- Sample components
Sample components
-Entire : Human beta-estradiol 17-(beta-D-glucuronide)-bound ABC transporte...
| Entire | Name: Human beta-estradiol 17-(beta-D-glucuronide)-bound ABC transporter ABCC3 in nanodiscs |
|---|---|
| Components |
|
-Supramolecule #1: Human beta-estradiol 17-(beta-D-glucuronide)-bound ABC transporte...
| Supramolecule | Name: Human beta-estradiol 17-(beta-D-glucuronide)-bound ABC transporter ABCC3 in nanodiscs type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 169.4 kDa/nm |
-Macromolecule #1: ATP-binding cassette sub-family C member 3
| Macromolecule | Name: ATP-binding cassette sub-family C member 3 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 169.504719 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDALCGSGEL GSKFWDSNLS VHTENPDLTP CFQNSLLAWV PCIYLWVALP CYLLYLRHHC RGYIILSHLS KLKMVLGVLL WCVSWADLF YSFHGLVHGR APAPVFFVTP LVVGVTMLLA TLLIQYERLQ GVQSSGVLII FWFLCVVCAI VPFRSKILLA K AEGEISDP ...String: MDALCGSGEL GSKFWDSNLS VHTENPDLTP CFQNSLLAWV PCIYLWVALP CYLLYLRHHC RGYIILSHLS KLKMVLGVLL WCVSWADLF YSFHGLVHGR APAPVFFVTP LVVGVTMLLA TLLIQYERLQ GVQSSGVLII FWFLCVVCAI VPFRSKILLA K AEGEISDP FRFTTFYIHF ALVLSALILA CFREKPPFFS AKNVDPNPYP ETSAGFLSRL FFWWFTKMAI YGYRHPLEEK DL WSLKEED RSQMVVQQLL EAWRKQEKQT ARHKASAAPG KNASGEDEVL LGARPRPRKP SFLKALLATF GSSFLISACF KLI QDLLSF INPQLLSILI RFISNPMAPS WWGFLVAGLM FLCSMMQSLI LQHYYHYIFV TGVKFRTGIM GVIYRKALVI TNSV KRAST VGEIVNLMSV DAQRFMDLAP FLNLLWSAPL QIILAIYFLW QNLGPSVLAG VAFMVLLIPL NGAVAVKMRA FQVKQ MKLK DSRIKLMSEI LNGIKVLKLY AWEPSFLKQV EGIRQGELQL LRTAAYLHTT TTFTWMCSPF LVTLITLWVY VYVDPN NVL DAEKAFVSVS LFNILRLPLN MLPQLISNLT QASVSLKRIQ QFLSQEELDP QSVERKTISP GYAITIHSGT FTWAQDL PP TLHSLDIQVP KGALVAVVGP VGCGKSSLVS ALLGEMEKLE GKVHMKGSVA YVPQQAWIQN CTLQENVLFG KALNPKRY Q QTLEACALLA DLEMLPGGDQ TEIGEKGINL SGGQRQRVSL ARAVYSDADI FLLDDPLSAV DSHVAKHIFD HVIGPEGVL AGKTRVLVTH GISFLPQTDF IIVLADGQVS EMGPYPALLQ RNGSFANFLC NYAPDEDQGH LEDSWTALEG AEDKEALLIE DTLSNHTDL TDNDPVTYVV QKQFMRQLSA LSSDGEGQGR PVPRRHLGPS EKVQVTEAKA DGALTQEEKA AIGTVELSVF W DYAKAVGL CTTLAICLLY VGQSAAAIGA NVWLSAWTND AMADSRQNNT SLRLGVYAAL GILQGFLVML AAMAMAAGGI QA ARVLHQA LLHNKIRSPQ SFFDTTPSGR ILNCFSKDIY VVDEVLAPVI LMLLNSFFNA ISTLVVIMAS TPLFTVVILP LAV LYTLVQ RFYAATSRQL KRLESVSRSP IYSHFSETVT GASVIRAYNR SRDFEIISDT KVDANQRSCY PYIISNRWLS IGVE FVGNC VVLFAALFAV IGRSSLNPGL VGLSVSYSLQ VTFALNWMIR MMSDLESNIV AVERVKEYSK TETEAPWVVE GSRPP EGWP PRGEVEFRNY SVRYRPGLDL VLRDLSLHVH GGEKVGIVGR TGAGKSSMTL CLFRILEAAK GEIRIDGLNV ADIGLH DLR SQLTIIPQDP ILFSGTLRMN LDPFGSYSEE DIWWALELSH LHTFVSSQPA GLDFQCSEGG ENLSVGQRQL VCLARAL LR KSRILVLDEA TAAIDLETDN LIQATIRTQF DTCTVLTIAH RLNTIMDYTR VLVLDKGVVA EFDSPANLIA ARGIFYGM A RDAGLA UniProtKB: ATP-binding cassette sub-family C member 3 |
-Macromolecule #2: Estradiol-17beta-glucuronide
| Macromolecule | Name: Estradiol-17beta-glucuronide / type: ligand / ID: 2 / Number of copies: 2 / Formula: U38 |
|---|---|
| Molecular weight | Theoretical: 448.506 Da |
| Chemical component information | 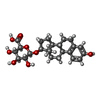 ChemComp-U38: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.85 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 54.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.65 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 177500 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)