+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | F8-A22-E4 complex in hexameric form local map | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | MPXV / complex / RECOMBINATION / REPLICATION | |||||||||
| Biological species |  Monkeypox virus Monkeypox virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Li YN / Shen YP / Hu ZW / Yan RH | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Structural basis for the assembly of the DNA polymerase holoenzyme from a monkeypox virus variant. Authors: Yaning Li / Yaping Shen / Ziwei Hu / Renhong Yan /  Abstract: The ongoing global pandemic caused by a variant of the monkeypox (or mpox) virus (MPXV) has prompted widespread concern. The MPXV DNA polymerase holoenzyme, consisting of F8, A22, and E4, is vital ...The ongoing global pandemic caused by a variant of the monkeypox (or mpox) virus (MPXV) has prompted widespread concern. The MPXV DNA polymerase holoenzyme, consisting of F8, A22, and E4, is vital for replicating the viral genome and represents a crucial target for the development of antiviral drugs. However, the assembly and working mechanism for the DNA polymerase holoenzyme of MPXV remains elusive. Here, we present the cryo-electron microscopy (cryo-EM) structure of the DNA polymerase holoenzyme at an overall resolution of 3.5 Å. Unexpectedly, the holoenzyme is assembled as a dimer of heterotrimers, of which the extra interface between the thumb domain of F8 and A22 shows a clash between A22 and substrate DNA, suggesting an autoinhibition state. Addition of exogenous double-stranded DNA shifts the hexamer into trimer exposing DNA binding sites, potentially representing a more active state. Our findings provide crucial steps toward developing targeted antiviral therapies for MPXV and related viruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34885.map.gz emd_34885.map.gz | 85.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34885-v30.xml emd-34885-v30.xml emd-34885.xml emd-34885.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34885.png emd_34885.png | 30.6 KB | ||
| Filedesc metadata |  emd-34885.cif.gz emd-34885.cif.gz | 3.8 KB | ||
| Others |  emd_34885_half_map_1.map.gz emd_34885_half_map_1.map.gz emd_34885_half_map_2.map.gz emd_34885_half_map_2.map.gz | 71.4 MB 71.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34885 http://ftp.pdbj.org/pub/emdb/structures/EMD-34885 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34885 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34885 | HTTPS FTP |
-Validation report
| Summary document |  emd_34885_validation.pdf.gz emd_34885_validation.pdf.gz | 761.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34885_full_validation.pdf.gz emd_34885_full_validation.pdf.gz | 761.4 KB | Display | |
| Data in XML |  emd_34885_validation.xml.gz emd_34885_validation.xml.gz | 12.9 KB | Display | |
| Data in CIF |  emd_34885_validation.cif.gz emd_34885_validation.cif.gz | 15.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34885 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34885 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34885 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34885 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34885.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34885.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.095 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_34885_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
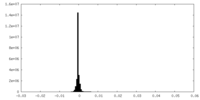
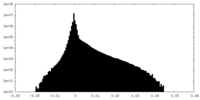
| Density Histograms |
-Half map: #2
| File | emd_34885_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
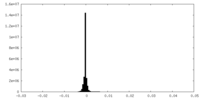
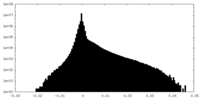
| Density Histograms |
- Sample components
Sample components
-Entire : F8-A22-E4 complex of MPXV in hexameric form
| Entire | Name: F8-A22-E4 complex of MPXV in hexameric form |
|---|---|
| Components |
|
-Supramolecule #1: F8-A22-E4 complex of MPXV in hexameric form
| Supramolecule | Name: F8-A22-E4 complex of MPXV in hexameric form / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Monkeypox virus Monkeypox virus |
-Supramolecule #2: DNA polymerase processivity factor component A20
| Supramolecule | Name: DNA polymerase processivity factor component A20 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Monkeypox virus Monkeypox virus |
-Supramolecule #3: E4R
| Supramolecule | Name: E4R / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Monkeypox virus Monkeypox virus |
-Supramolecule #4: DNA polymerase
| Supramolecule | Name: DNA polymerase / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #3 |
|---|
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.4000000000000001 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0.6) / Number images used: 383777 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)