[English] 日本語
 Yorodumi
Yorodumi- EMDB-34495: type I-B Cascade bound to a PAM-containing dsDNA target at 3.8 an... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | type I-B Cascade bound to a PAM-containing dsDNA target at 3.8 angstrom resolution. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Type I-B / CRISPR-Cas / Cascade / RNA BINDING PROTEIN | |||||||||
| Function / homology | CRISPR type MYXAN-associated protein Cmx8 / CRISPR-associated protein Cas5, N-terminal / defense response to virus / Uncharacterized protein / Fruiting body developmental protein R-like protein / Type I-MYXAN CRISPR-associated protein Cmx8 Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Xiao Y / Lu M / Yu C / Zhang Y | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structure and genome editing of type I-B CRISPR-Cas. Authors: Meiling Lu / Chenlin Yu / Yuwen Zhang / Wenjun Ju / Zhi Ye / Chenyang Hua / Jinze Mao / Chunyi Hu / Zhenhuang Yang / Yibei Xiao /   Abstract: Type I CRISPR-Cas systems employ multi-subunit effector Cascade and helicase-nuclease Cas3 to target and degrade foreign nucleic acids, representing the most abundant RNA-guided adaptive immune ...Type I CRISPR-Cas systems employ multi-subunit effector Cascade and helicase-nuclease Cas3 to target and degrade foreign nucleic acids, representing the most abundant RNA-guided adaptive immune systems in prokaryotes. Their ability to cause long fragment deletions have led to increasing interests in eukaryotic genome editing. While the Cascade structures of all other six type I systems have been determined, the structure of the most evolutionarily conserved type I-B Cascade is still missing. Here, we present two cryo-EM structures of the Synechocystis sp. PCC 6714 (Syn) type I-B Cascade, revealing the molecular mechanisms that underlie RNA-directed Cascade assembly, target DNA recognition, and local conformational changes of the effector complex upon R-loop formation. Remarkably, a loop of Cas5 directly intercalated into the major groove of the PAM and facilitated PAM recognition. We further characterized the genome editing profiles of this I-B Cascade-Cas3 in human CD3 T cells using mRNA-mediated delivery, which led to unidirectional 4.5 kb deletion in TRAC locus and achieved an editing efficiency up to 41.2%. Our study provides the structural basis for understanding target DNA recognition by type I-B Cascade and lays foundation for harnessing this system for long range genome editing in human T cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34495.map.gz emd_34495.map.gz | 51.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34495-v30.xml emd-34495-v30.xml emd-34495.xml emd-34495.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34495.png emd_34495.png | 58.9 KB | ||
| Filedesc metadata |  emd-34495.cif.gz emd-34495.cif.gz | 6.5 KB | ||
| Others |  emd_34495_half_map_1.map.gz emd_34495_half_map_1.map.gz emd_34495_half_map_2.map.gz emd_34495_half_map_2.map.gz | 95.5 MB 95.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34495 http://ftp.pdbj.org/pub/emdb/structures/EMD-34495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34495 | HTTPS FTP |
-Validation report
| Summary document |  emd_34495_validation.pdf.gz emd_34495_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34495_full_validation.pdf.gz emd_34495_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_34495_validation.xml.gz emd_34495_validation.xml.gz | 13.5 KB | Display | |
| Data in CIF |  emd_34495_validation.cif.gz emd_34495_validation.cif.gz | 15.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34495 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34495 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34495 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34495 | HTTPS FTP |
-Related structure data
| Related structure data |  8h67MC  8ip0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34495.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34495.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34495_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
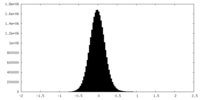
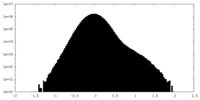
| Density Histograms |
-Half map: #1
| File | emd_34495_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
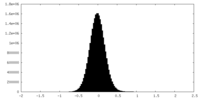
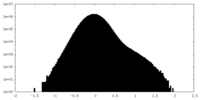
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of Synechocystis sp. PCC6714 Cascade bound to a...
| Entire | Name: Cryo-EM structure of Synechocystis sp. PCC6714 Cascade bound to a PAM-containing dsDNA target at 3.8 angstrom resolution. |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of Synechocystis sp. PCC6714 Cascade bound to a...
| Supramolecule | Name: Cryo-EM structure of Synechocystis sp. PCC6714 Cascade bound to a PAM-containing dsDNA target at 3.8 angstrom resolution. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: CRISPR RNA
| Macromolecule | Name: CRISPR RNA / type: rna / ID: 1 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 22.64632 KDa |
| Sequence | String: UGAGCACUUU AUCACCGUGU CCCCAAUCUG GAUAUUUUGU GUGUGUCCAA ACCAUUGAUG CCGUAAGGCG U GENBANK: GENBANK: CP007544.1 |
-Macromolecule #2: Target DNA
| Macromolecule | Name: Target DNA / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 3.390262 KDa |
| Sequence | String: (DA)(DT)(DA)(DA)(DA)(DC)(DA)(DT)(DG)(DG) (DA) |
-Macromolecule #3: Non target DNA
| Macromolecule | Name: Non target DNA / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 2.705797 KDa |
| Sequence | String: (DT)(DC)(DC)(DA)(DT)(DG)(DT)(DT)(DA) |
-Macromolecule #4: CRISPR associated protein Cas5
| Macromolecule | Name: CRISPR associated protein Cas5 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 26.592424 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAQLALALDT VTRYLRLKAP FAAFRPFQSG SFRSTTPVPS FSAVYGLLLN LAGIEQRQEV EGKVTLIKPK AELPKLAIAI GQVKPSSTS LINQQLHNYP VGNSGKEFAS RTFGSKYWIA PVRREVLVNL DLIIGLQSPV EFWQKLDQGL KGETVINRYG L PFAGDNNF ...String: MAQLALALDT VTRYLRLKAP FAAFRPFQSG SFRSTTPVPS FSAVYGLLLN LAGIEQRQEV EGKVTLIKPK AELPKLAIAI GQVKPSSTS LINQQLHNYP VGNSGKEFAS RTFGSKYWIA PVRREVLVNL DLIIGLQSPV EFWQKLDQGL KGETVINRYG L PFAGDNNF LFDEIYPIEK PDLASWYCPL EPDTRPNQGA CRLTLWIDRE NNTQTTIKVF SPSDFRLEPP AKAWQQLPG UniProtKB: Uncharacterized protein |
-Macromolecule #5: CRISPR associated protein Cas7
| Macromolecule | Name: CRISPR associated protein Cas7 / type: protein_or_peptide / ID: 5 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 33.789074 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSNLNLFATI LTYPAPASNY RGESEENRSV IQKILKDGQK YAIISPESMR NALREMLIEL GQPNNRTRLH SEDQLAVEFK EYPNPDKFA DDFLFGYMVA QTNDAKEMKK LNRPAKRDSI FRCNMAVAVN PYKYDTVFYQ SPLNAGDSAW KNSTSSALLH R EVTHTAFQ ...String: MSNLNLFATI LTYPAPASNY RGESEENRSV IQKILKDGQK YAIISPESMR NALREMLIEL GQPNNRTRLH SEDQLAVEFK EYPNPDKFA DDFLFGYMVA QTNDAKEMKK LNRPAKRDSI FRCNMAVAVN PYKYDTVFYQ SPLNAGDSAW KNSTSSALLH R EVTHTAFQ YPFALAGKDC AAKPEWVKAL LQAIAELNGV AGGHARAYYE FAPRSVVARL TPKLVAGYQT YGFDAEGNWL EL SRLTATD SDNLDLPANE FWLGGELVRK MDQEQKAQLE AMGAHLYANP EKLFADLADS FLGV UniProtKB: Fruiting body developmental protein R-like protein |
-Macromolecule #6: CRISPR associated protein Cas8
| Macromolecule | Name: CRISPR associated protein Cas8 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 69.287305 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH HHSQWSHPQF EKGGGSGGGS GGSAWSHPQF EKLEVLFQGP GSMPKTQAEI LTLDFNLAEL PSAQHRAGLA GLILMIREL KKWPWFKIRQ KEKDVLLSIE NLDQYGASIQ LNLEGLIALF DLAYLSFTEE RKSKSKIKDF KRVDEIEIEE N GKNKIQKY ...String: MGSSHHHHHH HHSQWSHPQF EKGGGSGGGS GGSAWSHPQF EKLEVLFQGP GSMPKTQAEI LTLDFNLAEL PSAQHRAGLA GLILMIREL KKWPWFKIRQ KEKDVLLSIE NLDQYGASIQ LNLEGLIALF DLAYLSFTEE RKSKSKIKDF KRVDEIEIEE N GKNKIQKY YFYDVITPQG GFLAGWDKSD GQIWLRIWRD MFWSIIKGVP ATRNPFNNRC GLNLNAGDSF SKDVESVWKS LQ NAEKTTG QSGAFYLGAM AVNAENVSTD DLIKWQFLLH FWAFVAQVYC PYILDKDGKR NFNGYVIVIP DIANLEDFCD ILP DVLSNR NSKAFGFRPQ ESVIDVPEQG ALELLNLIKQ RIAKKAGSGL LSDLIVGVEV IHAEKQGNSI KLHSVSYLQP NEES VDDYN AIKNSYYCPW FRRQLLLNLV NPKFDLASQS WLKRHPWYGF GDLLSRIPQR WLKENNSYFS HDARQLFTQK GDFDM TVAT TKTREYAEIV YKIAQGFVLS KLSSKHDLQW SKCKGNPKLE REYNDKKEKV VNEAFLAIRS RTEKQAFIDY FVSTLY PHV RQDEFVDFAQ KLFQDTDEIR SLTLLALSSQ YPIKRQGETE UniProtKB: Type I-MYXAN CRISPR-associated protein Cmx8 |
-Macromolecule #7: CRISPR associated protein Cas11b
| Macromolecule | Name: CRISPR associated protein Cas11b / type: protein_or_peptide / ID: 7 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 14.5385 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTVATTKTRE YAEIVYKIAQ GFVLSKLSSK HDLQWSKCKG NPKLEREYND KKEKVVNEAF LAIRSRTEKQ AFIDYFVSTL YPHVRQDEF VDFAQKLFQD TDEIRSLTLL ALSSQYPIKR QGETE UniProtKB: Type I-MYXAN CRISPR-associated protein Cmx8 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.1 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 33011 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: OTHER |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)