+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Cas7-11-crRNA-Csx29 ternary complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cas7-11 / crRNA / CRISPR-Cas / Csx29 / RNA BINDING PROTEIN | |||||||||
| Function / homology | CHAT domain / CHAT domain / : / CRISPR type III-associated protein / RAMP superfamily / defense response to virus / CRISPR-associated RAMP family protein / CHAT domain-containing protein Function and homology information Function and homology information | |||||||||
| Biological species |  Desulfonema ishimotonii (bacteria) Desulfonema ishimotonii (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.29 Å | |||||||||
 Authors Authors | Huo Y / Dong Q / Zhao H / Jiang T | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2023 Journal: Nat Microbiol / Year: 2023Title: Cryo-EM structure and protease activity of the type III-E CRISPR-Cas effector. Authors: Yangao Huo / Hongshen Zhao / Qinghua Dong / Tao Jiang /  Abstract: The recently discovered type III-E CRISPR-Cas effector Cas7-11 shows promise when used as an RNA manipulation tool, but its structure and the mechanisms underlying its function remain unclear. Here ...The recently discovered type III-E CRISPR-Cas effector Cas7-11 shows promise when used as an RNA manipulation tool, but its structure and the mechanisms underlying its function remain unclear. Here we present four cryo-EM structures of Desulfonema ishimotonii Cas7-11-crRNA complex in pre-target and target RNA-bound states, and the cryo-EM structure of DiCas7-11-crRNA bound to its accessory protein DiCsx29. These data reveal structural elements for pre-crRNA processing, target RNA cleavage and regulation. Moreover, a 3' seed region of crRNA is involved in regulating RNA cleavage activity of DiCas7-11-crRNA-Csx29. Our analysis also shows that both the minimal mismatch of 4 nt to the 5' handle of crRNA and the minimal matching of the first 12 nt of the spacer by the target RNA are essential for triggering the protease activity of DiCas7-11-crRNA-Csx29 towards DiCsx30. Taken together, we propose that target RNA recognition and cleavage regulate and fine-tune the protease activity of DiCas7-11-crRNA-Csx29, thus preventing auto-immune responses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33959.map.gz emd_33959.map.gz | 166.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33959-v30.xml emd-33959-v30.xml emd-33959.xml emd-33959.xml | 16.6 KB 16.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33959.png emd_33959.png | 107.6 KB | ||
| Filedesc metadata |  emd-33959.cif.gz emd-33959.cif.gz | 6.7 KB | ||
| Others |  emd_33959_half_map_1.map.gz emd_33959_half_map_1.map.gz emd_33959_half_map_2.map.gz emd_33959_half_map_2.map.gz | 140.7 MB 140.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33959 http://ftp.pdbj.org/pub/emdb/structures/EMD-33959 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33959 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33959 | HTTPS FTP |
-Validation report
| Summary document |  emd_33959_validation.pdf.gz emd_33959_validation.pdf.gz | 844.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33959_full_validation.pdf.gz emd_33959_full_validation.pdf.gz | 843.7 KB | Display | |
| Data in XML |  emd_33959_validation.xml.gz emd_33959_validation.xml.gz | 14.8 KB | Display | |
| Data in CIF |  emd_33959_validation.cif.gz emd_33959_validation.cif.gz | 17.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33959 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33959 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33959 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33959 | HTTPS FTP |
-Related structure data
| Related structure data |  7yndMC  7yn9C  7ynaC  7ynbC  7yncC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33959.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33959.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_33959_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
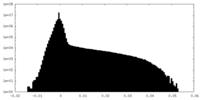
| Density Histograms |
-Half map: #1
| File | emd_33959_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
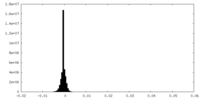
| Density Histograms |
- Sample components
Sample components
-Entire : Cas7-11-crRNA-Csx29 ternary complex
| Entire | Name: Cas7-11-crRNA-Csx29 ternary complex |
|---|---|
| Components |
|
-Supramolecule #1: Cas7-11-crRNA-Csx29 ternary complex
| Supramolecule | Name: Cas7-11-crRNA-Csx29 ternary complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Desulfonema ishimotonii (bacteria) Desulfonema ishimotonii (bacteria) |
-Macromolecule #1: CRISPR-associated RAMP family protein
| Macromolecule | Name: CRISPR-associated RAMP family protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Desulfonema ishimotonii (bacteria) Desulfonema ishimotonii (bacteria) |
| Molecular weight | Theoretical: 183.933 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTTTMKISIE FLEPFRMTKW QESTRRNKNN KEFVRGQAFA RWHRNKKDNT KGRPYITGTL LRSAVIRSAE NLLTLSDGKI SEKTCCPGK FDTEDKDRLL QLRQRSTLRW TDKNPCPDNA ETYCPFCELL GRSGNDGKKA EKKDWRFRIH FGNLSLPGKP D FDGPKAIG ...String: MTTTMKISIE FLEPFRMTKW QESTRRNKNN KEFVRGQAFA RWHRNKKDNT KGRPYITGTL LRSAVIRSAE NLLTLSDGKI SEKTCCPGK FDTEDKDRLL QLRQRSTLRW TDKNPCPDNA ETYCPFCELL GRSGNDGKKA EKKDWRFRIH FGNLSLPGKP D FDGPKAIG SQRVLNRVDF KSGKAHDFFK AYEVDHTRFP RFEGEITIDN KVSAEARKLL CDSLKFTDRL CGALCVIRFD EY TPAADSG KQTENVQAEP NANLAEKTAE QIISILDDNK KTEYTRLLAD AIRSLRRSSK LVAGLPKDHD GKDDHYLWDI GKK KKDENS VTIRQILTTS ADTKELKNAG KWREFCEKLG EALYLKSKDM SGGLKITRRI LGDAEFHGKP DRLEKSRSVS IGSV LKETV VCGELVAKTP FFFGAIDEDA KQTDLQVLLT PDNKYRLPRS AVRGILRRDL QTYFDSPCNA ELGGRPCMCK TCRIM RGIT VMDARSEYNA PPEIRHRTRI NPFTGTVAEG ALFNMEVAPE GIVFPFQLRY RGSEDGLPDA LKTVLKWWAE GQAFMS GAA STGKGRFRME NAKYETLDLS DENQRNDYLK NWGWRDEKGL EELKKRLNSG LPEPGNYRDP KWHEINVSIE MASPFIN GD PIRAAVDKRG TDVVTFVKYK AEGEEAKPVC AYKAESFRGV IRSAVARIHM EDGVPLTELT HSDCECLLCQ IFGSEYEA G KIRFEDLVFE SDPEPVTFDH VAIDRFTGGA ADKKKFDDSP LPGSPARPLM LKGSFWIRRD VLEDEEYCKA LGKALADVN NGLYPLGGKS AIGYGQVKSL GIKGDDKRIS RLMNPAFDET DVAVPEKPKT DAEVRIEAEK VYYPHYFVEP HKKVEREEKP CGHQKFHEG RLTGKIRCKL ITKTPLIVPD TSNDDFFRPA DKEARKEKDE YHKSYAFFRL HKQIMIPGSE LRGMVSSVYE T VTNSCFRI FDETKRLSWR MDADHQNVLQ DFLPGRVTAD GKHIQKFSET ARVPFYDKTQ KHFDILDEQE IAGEKPVRMW VK RFIKRLS LVDPAKHPQK KQDNKWKRRK EGIATFIEQK NGSYYFNVVT NNGCTSFHLW HKPDNFDQEK LEGIQNGEKL DCW VRDSRY QKAFQEIPEN DPDGWECKEG YLHVVGPSKV EFSDKKGDVI NNFQGTLPSV PNDWKTIRTN DFKNRKRKNE PVFC CEDDK GNYYTMAKYC ETFFFDLKEN EEYEIPEKAR IKYKELLRVY NNNPQAVPES VFQSRVAREN VEKLKSGDLV YFKHN EKYV EDIVPVRISR TVDDRMIGKR MSADLRPCHG DWVEDGDLSA LNAYPEKRLL LRHPKGLCPA CRLFGTGSYK GRVRFG FAS LENDPEWLIP GKNPGDPFHG GPVMLSLLER PRPTWSIPGS DNKFKVPGRK FYVHHHAWKT IKDGNHPTTG KAIEQSP NN RTVEALAGGN SFSFEIAFEN LKEWELGLLI HSLQLEKGLA HKLGMAKSMG FGSVEIDVES VRLRKDWKQW RNGNSEIP N WLGKGFAKLK EWFRDELDFI ENLKKLLWFP EGDQAPRVCY PMLRKKDDPN GNSGYEELKD GEFKKEDRQK KLTTPWTPW A UniProtKB: CRISPR-associated RAMP family protein |
-Macromolecule #3: CHAT domain-containing protein
| Macromolecule | Name: CHAT domain-containing protein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Desulfonema ishimotonii (bacteria) Desulfonema ishimotonii (bacteria) |
| Molecular weight | Theoretical: 88.311875 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSNPIRDIQD RLKTAKFDNK DDMMNLASSL YKYEKQLMDS SEATLCQQGL SNRPNSFSQL SQFRDSDIQS KAGGQTGKFW QNEYEACKN FQTHKERRET LEQIIRFLQN GAEEKDADDL LLKTLARAYF HRGLLYRPKG FSVPARKVEA MKKAIAYCEI I LDKNEEES ...String: MSNPIRDIQD RLKTAKFDNK DDMMNLASSL YKYEKQLMDS SEATLCQQGL SNRPNSFSQL SQFRDSDIQS KAGGQTGKFW QNEYEACKN FQTHKERRET LEQIIRFLQN GAEEKDADDL LLKTLARAYF HRGLLYRPKG FSVPARKVEA MKKAIAYCEI I LDKNEEES EALRIWLYAA MELRRCGEEY PENFAEKLFY LANDGFISEL YDIRLFLEYT EREEDNNFLD MILQENQDRE RL FELCLYK ARACFHLNQL NDVRIYGESA IDNAPGAFAD PFWDELVEFI RMLRNKKSEL WKEIAIKAWD KCREKEMKVG NNI YLSWYW ARQRELYDLA FMAQDGIEKK TRIADSLKSR TTLRIQELNE LRKDAHRKQN RRLEDKLDRI IEQENEARDG AYLR RNPPC FTGGKREEIP FARLPQNWIA VHFYLNELES HEGGKGGHAL IYDPQKAEKD QWQDKSFDYK ELHRKFLEWQ ENYIL NEEG SADFLVTLCR EIEKAMPFLF KSEVIPEDRP VLWIPHGFLH RLPLHAAMKS GNNSNIEIFW ERHASRYLPA WHLFDP APY SREESSTLLK NFEEYDFQNL ENGEIEVYAP SSPKKVKEAI RENPAILLLL CHGEADMTNP FRSCLKLKNK DMTIFDL LT VEDVRLSGSR ILLGACESDM VPPLEFSVDE HLSVSGAFLS HKAGEIVAGL WTVDSEKVDE CYSYLVEEKD FLRNLQEW Q MAETENFRSE NDSSLFYKIA PFRIIGFPAE UniProtKB: CHAT domain-containing protein |
-Macromolecule #2: crRNA (38-MER)
| Macromolecule | Name: crRNA (38-MER) / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Desulfonema ishimotonii (bacteria) Desulfonema ishimotonii (bacteria) |
| Molecular weight | Theoretical: 15.094032 KDa |
| Sequence | String: UUGAUGUCAC GGAACAGGAA CUUGAACAAC AUCGUUACUA ACGAGCU |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.29 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 389783 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)