[English] 日本語
 Yorodumi
Yorodumi- EMDB-29609: Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of chimeric Sec complex (human Sec61 and yeast Sec63-71-72) inhibited by cotransin | |||||||||
 Map data Map data | Unsharpened map for cotransin bound chimeric Sec complex (whole). | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | translocon / inhibitor / protein translocation / PROTEIN TRANSPORT / PROTEIN TRANSPORT-INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationSec62/Sec63 complex / translocon complex / cytosol to endoplasmic reticulum transport / endoplasmic reticulum Sec complex / pronephric nephron development / rough endoplasmic reticulum membrane / endoplasmic reticulum quality control compartment / cotranslational protein targeting to membrane / Ssh1 translocon complex / Sec61 translocon complex ...Sec62/Sec63 complex / translocon complex / cytosol to endoplasmic reticulum transport / endoplasmic reticulum Sec complex / pronephric nephron development / rough endoplasmic reticulum membrane / endoplasmic reticulum quality control compartment / cotranslational protein targeting to membrane / Ssh1 translocon complex / Sec61 translocon complex / protein targeting to ER / protein insertion into ER membrane / post-translational protein targeting to endoplasmic reticulum membrane / filamentous growth / SRP-dependent cotranslational protein targeting to membrane, translocation / SRP-dependent cotranslational protein targeting to membrane / signal sequence binding / post-translational protein targeting to membrane, translocation / endoplasmic reticulum organization / nuclear inner membrane / epidermal growth factor binding / retrograde protein transport, ER to cytosol / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / protein transmembrane transporter activity / SRP-dependent cotranslational protein targeting to membrane / ERAD pathway / guanyl-nucleotide exchange factor activity / cell periphery / calcium channel activity / ribosome binding / ER-Phagosome pathway / endoplasmic reticulum membrane / endoplasmic reticulum / mitochondrion / RNA binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.77 Å | |||||||||
 Authors Authors | Park E / Itskanov S | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2023 Journal: Nat Chem Biol / Year: 2023Title: A common mechanism of Sec61 translocon inhibition by small molecules. Authors: Samuel Itskanov / Laurie Wang / Tina Junne / Rumi Sherriff / Li Xiao / Nicolas Blanchard / Wei Q Shi / Craig Forsyth / Dominic Hoepfner / Martin Spiess / Eunyong Park /    Abstract: The Sec61 complex forms a protein-conducting channel in the endoplasmic reticulum membrane that is required for secretion of soluble proteins and production of many membrane proteins. Several natural ...The Sec61 complex forms a protein-conducting channel in the endoplasmic reticulum membrane that is required for secretion of soluble proteins and production of many membrane proteins. Several natural and synthetic small molecules specifically inhibit Sec61, generating cellular effects that are useful for therapeutic purposes, but their inhibitory mechanisms remain unclear. Here we present near-atomic-resolution structures of human Sec61 inhibited by a comprehensive panel of structurally distinct small molecules-cotransin, decatransin, apratoxin, ipomoeassin, mycolactone, cyclotriazadisulfonamide and eeyarestatin. All inhibitors bind to a common lipid-exposed pocket formed by the partially open lateral gate and plug domain of Sec61. Mutations conferring resistance to the inhibitors are clustered at this binding pocket. The structures indicate that Sec61 inhibitors stabilize the plug domain in a closed state, thereby preventing the protein-translocation pore from opening. Our study provides the atomic details of Sec61-inhibitor interactions and the structural framework for further pharmacological studies and drug design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29609.map.gz emd_29609.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29609-v30.xml emd-29609-v30.xml emd-29609.xml emd-29609.xml | 25.5 KB 25.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29609.png emd_29609.png | 66.7 KB | ||
| Others |  emd_29609_additional_1.map.gz emd_29609_additional_1.map.gz emd_29609_half_map_1.map.gz emd_29609_half_map_1.map.gz emd_29609_half_map_2.map.gz emd_29609_half_map_2.map.gz | 32.3 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29609 http://ftp.pdbj.org/pub/emdb/structures/EMD-29609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29609 | HTTPS FTP |
-Validation report
| Summary document |  emd_29609_validation.pdf.gz emd_29609_validation.pdf.gz | 886.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29609_full_validation.pdf.gz emd_29609_full_validation.pdf.gz | 886 KB | Display | |
| Data in XML |  emd_29609_validation.xml.gz emd_29609_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_29609_validation.cif.gz emd_29609_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29609 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29609 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29609 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29609 | HTTPS FTP |
-Related structure data
| Related structure data |  8dnvC  8dnwC  8dnxC  8dnyC  8dnzC  8do0C  8do1C  8do2C  8do3C C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29609.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29609.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map for cotransin bound chimeric Sec complex (whole). | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
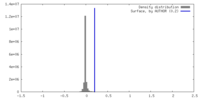
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Sharpened map for cotransin bound chimeric Sec complex (whole).
| File | emd_29609_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map for cotransin bound chimeric Sec complex (whole). | ||||||||||||
| Projections & Slices |
| ||||||||||||
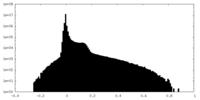
| Density Histograms |
-Half map: Half map B for cotransin bound chimeric Sec complex (whole).
| File | emd_29609_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B for cotransin bound chimeric Sec complex (whole). | ||||||||||||
| Projections & Slices |
| ||||||||||||
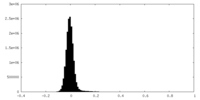
| Density Histograms |
-Half map: Half map A for cotransin bound chimeric Sec complex (whole).
| File | emd_29609_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A for cotransin bound chimeric Sec complex (whole). | ||||||||||||
| Projections & Slices |
| ||||||||||||
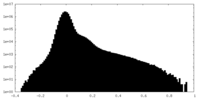
| Density Histograms |
- Sample components
Sample components
-Entire : A human-yeast chimeric Sec complex treated with cotransin
| Entire | Name: A human-yeast chimeric Sec complex treated with cotransin |
|---|---|
| Components |
|
-Supramolecule #1: A human-yeast chimeric Sec complex treated with cotransin
| Supramolecule | Name: A human-yeast chimeric Sec complex treated with cotransin type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organelle: endoplasmic reticulum Homo sapiens (human) / Organelle: endoplasmic reticulum |
-Macromolecule #1: Protein transport protein Sec61 subunit gamma
| Macromolecule | Name: Protein transport protein Sec61 subunit gamma / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MDQVMQFVEP SRQFVKDSIR LVKRCTKPDR KEFQKIAMAT AIGFAIMGFI GFFVKLIHIP INNIIVGG UniProtKB: Protein transport protein Sec61 subunit gamma |
-Macromolecule #2: Protein transport protein Sec61 subunit beta
| Macromolecule | Name: Protein transport protein Sec61 subunit beta / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MPGPTPSGTN VGSSGRSPSK AVAARAAGST VRQRKNASCG TRSAGRTTSA GTGGMWRFYT EDSPGLKVGP VPVLVMSLLF IASVFMLHI WGKYTRS UniProtKB: Protein transport protein Sec61 subunit beta |
-Macromolecule #3: Protein transport protein Sec61 subunit alpha isoform 1
| Macromolecule | Name: Protein transport protein Sec61 subunit alpha isoform 1 type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MAIKFLEVIK PFCVILPEIQ KPERKIQFKE KVLWTAITLF IFLVCCQIPL FGIMSSDSAD PFYWMRVILA SNRGTLMELG ISPIVTSGL IMQLLAGAKI IEVGDTPKDR ALFNGAQKLF GMIITIGQSI VYVMTGMYGD PSEMGAGICL LITIQLFVAG L IVLLLDEL ...String: MAIKFLEVIK PFCVILPEIQ KPERKIQFKE KVLWTAITLF IFLVCCQIPL FGIMSSDSAD PFYWMRVILA SNRGTLMELG ISPIVTSGL IMQLLAGAKI IEVGDTPKDR ALFNGAQKLF GMIITIGQSI VYVMTGMYGD PSEMGAGICL LITIQLFVAG L IVLLLDEL LQKGYGLGSG ISLFIATNIC ETIVWKAFSP TTVNTGRGME FEGAIIALFH LLATRTDKVR ALREAFYRQN LP NLMNLIA TIFVFAVVIY FQGFRYELPI RSTKVRGQIG IYPIKLFYTS NIPIILQSAL VSNLYVISQM LSARFSGNLL VSL LGTWSD TSSGGPARAY PVGGLCYYLS PPESFGSVLE DPVHAVVYIV FMLGSCAFFS KTWIEVSGSS PRDIAKQFKD QGMV INGKR ETSIYRELKK IIPTAAAFGG LCIGALSVLA DFLGAIGSGT GILLAVTIIY QYFEIFVKEQ SEVGSMGALL F UniProtKB: Protein transport protein Sec61 subunit alpha isoform 1 |
-Macromolecule #4: Protein transport protein Sec71
| Macromolecule | Name: Protein transport protein Sec71 / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MSEFNETKFS NNGTFFETEE PIVETKSISV YTPLIYVFIL VVSLVMFASS YRKKQAKKIS EQPSIFDEND AHDLYFQIKE MSENEKIHEK VLKAALLNRG AESVRRSLKL KELAPQINLL YKNGSIGEDY WKRFETEVKL IELEFKDTLQ EAERLQPGWV QLFVMVCKEI ...String: MSEFNETKFS NNGTFFETEE PIVETKSISV YTPLIYVFIL VVSLVMFASS YRKKQAKKIS EQPSIFDEND AHDLYFQIKE MSENEKIHEK VLKAALLNRG AESVRRSLKL KELAPQINLL YKNGSIGEDY WKRFETEVKL IELEFKDTLQ EAERLQPGWV QLFVMVCKEI CFNQALSRRY QSILKRKEVC IKEWELKINN DGRLVN UniProtKB: Translocation protein SEC66 |
-Macromolecule #5: Protein transport protein Sec72
| Macromolecule | Name: Protein transport protein Sec72 / type: protein_or_peptide / ID: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MVTLEYNANS KLITASDAVV ALSTETNIDQ INVLTTSLIG ETNPNFTPQP NEALSKMIKG LFESGMKNLQ QKKLNEALKN VSLAIEMAQR KRAPWEAFAI QLPELHFMLR SKIDLCLILG KHLEALQDLD FLLGTGLIQP DVFVRKADCL LKLRQWEEAR ATCERGLALA PEDMKLRALL IETARNLAEY NGE UniProtKB: Translocation protein SEC72 |
-Macromolecule #6: Protein transport protein Sec63, yeast-human chimera
| Macromolecule | Name: Protein transport protein Sec63, yeast-human chimera / type: protein_or_peptide / ID: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: MAGQQFQYDD SGNTFFYFLT SFVGLIVIPM TLLQIYQIFF GANAEDGNSG KSKEFNEEVF KNLNEEYTSD EIKQFRRKFD KNSNKKSKIW SRRNIVLLAG WALFLFLAYK VSKTDREYQE YNPYEVLNLD PGATVAEIKK QYRLLSLKYH PDKGGDEVMF MRIAKAYAAL ...String: MAGQQFQYDD SGNTFFYFLT SFVGLIVIPM TLLQIYQIFF GANAEDGNSG KSKEFNEEVF KNLNEEYTSD EIKQFRRKFD KNSNKKSKIW SRRNIVLLAG WALFLFLAYK VSKTDREYQE YNPYEVLNLD PGATVAEIKK QYRLLSLKYH PDKGGDEVMF MRIAKAYAAL TDEESRKNWE EFGNPDGPQA TSFGIALPAW IVDQKNSILV LLVYGLAFMV ILPVVVGSWW YRTQSYTKKG IHNVTASNFV SNLVNYKPSE IVTTDLILHW LSFAHEFKQF FPDLQPTDFE KLLQDHINRR DSGKLNNAKF RIVAKCHSLL HGLLDIACGF RNLDIALGAI NTFKCIVQAV PLTPNCQILQ LPNVDKEHFI TKTGDIHTLG KLFTLEDAKI GEVLGIKDQA KLNETLRVAS HIPNLKIIKA DFLVPGENQV TPSSTPYISL KVLVRSAKQP LIPTSLIPEE NLTEPQDFES QRDPFAMMSK QPLVPYSFAP FFPTKRRGSW CCLVSSQKDG KILQTPIIIE KLSYKNLNDD KDFFDKRIKM DLTKHEKFDI NDWEIGTIKI PLGQPAPETV GDFFFRVIVK STDYFTTDLD ITMNMKVRDS PAVEQVEVYS EEDDEYSTDD DETESDDESD ASDYTDIDTD TEAEDDESPE GAGSNSLEVL FQ UniProtKB: Protein translocation protein SEC63 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 35 sec. / Pretreatment - Atmosphere: AIR / Details: Used a PELCO easiGlow glow discharge cleaner | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 4 seconds before plunging. | ||||||||||||||||||
| Details | Reconsitituted into a peptidisc. Monodisperse peak from a Superose 6 column. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)