[English] 日本語
 Yorodumi
Yorodumi- EMDB-29353: Structure of Agrobacterium tumefaciens bacteriophage Milano curve... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Agrobacterium tumefaciens bacteriophage Milano curved tail | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Myophage / redox trigger / VIRUS | |||||||||
| Function / homology | Tail sheath protein / Virion-associated protein Function and homology information Function and homology information | |||||||||
| Biological species |  Agrobacterium phage Milano (virus) Agrobacterium phage Milano (virus) | |||||||||
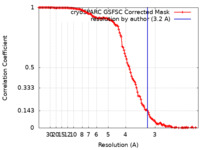
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Sonani RR / Leiman PG / Wang F / Kreutzberger MAB / Sebastian A / Esteves NC / Kelly RJ / Scharf B / Egelman EH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: An extensive disulfide bond network prevents tail contraction in Agrobacterium tumefaciens phage Milano. Authors: Ravi R Sonani / Lee K Palmer / Nathaniel C Esteves / Abigail A Horton / Amanda L Sebastian / Rebecca J Kelly / Fengbin Wang / Mark A B Kreutzberger / William K Russell / Petr G Leiman / ...Authors: Ravi R Sonani / Lee K Palmer / Nathaniel C Esteves / Abigail A Horton / Amanda L Sebastian / Rebecca J Kelly / Fengbin Wang / Mark A B Kreutzberger / William K Russell / Petr G Leiman / Birgit E Scharf / Edward H Egelman /  Abstract: A contractile sheath and rigid tube assembly is a widespread apparatus used by bacteriophages, tailocins, and the bacterial type VI secretion system to penetrate cell membranes. In this mechanism, ...A contractile sheath and rigid tube assembly is a widespread apparatus used by bacteriophages, tailocins, and the bacterial type VI secretion system to penetrate cell membranes. In this mechanism, contraction of an external sheath powers the motion of an inner tube through the membrane. The structure, energetics, and mechanism of the machinery imply rigidity and straightness. The contractile tail of Agrobacterium tumefaciens bacteriophage Milano is flexible and bent to varying degrees, which sets it apart from other contractile tail-like systems. Here, we report structures of the Milano tail including the sheath-tube complex, baseplate, and putative receptor-binding proteins. The flexible-to-rigid transformation of the Milano tail upon contraction can be explained by unique electrostatic properties of the tail tube and sheath. All components of the Milano tail, including sheath subunits, are crosslinked by disulfides, some of which must be reduced for contraction to occur. The putative receptor-binding complex of Milano contains a tailspike, a tail fiber, and at least two small proteins that form a garland around the distal ends of the tailspikes and tail fibers. Despite being flagellotropic, Milano lacks thread-like tail filaments that can wrap around the flagellum, and is thus likely to employ a different binding mechanism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29353.map.gz emd_29353.map.gz | 203.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29353-v30.xml emd-29353-v30.xml emd-29353.xml emd-29353.xml | 15 KB 15 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29353_fsc.xml emd_29353_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_29353.png emd_29353.png | 60.3 KB | ||
| Filedesc metadata |  emd-29353.cif.gz emd-29353.cif.gz | 5.5 KB | ||
| Others |  emd_29353_half_map_1.map.gz emd_29353_half_map_1.map.gz emd_29353_half_map_2.map.gz emd_29353_half_map_2.map.gz | 200.7 MB 200.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29353 http://ftp.pdbj.org/pub/emdb/structures/EMD-29353 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29353 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29353 | HTTPS FTP |
-Validation report
| Summary document |  emd_29353_validation.pdf.gz emd_29353_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29353_full_validation.pdf.gz emd_29353_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_29353_validation.xml.gz emd_29353_validation.xml.gz | 21.7 KB | Display | |
| Data in CIF |  emd_29353_validation.cif.gz emd_29353_validation.cif.gz | 28.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29353 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29353 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29353 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29353 | HTTPS FTP |
-Related structure data
| Related structure data |  8fopMC  8fouC  8foyC  8fqcC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_29353.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29353.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29353_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
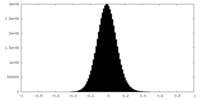
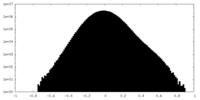
| Density Histograms |
-Half map: #2
| File | emd_29353_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
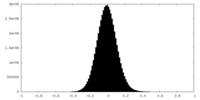
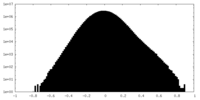
| Density Histograms |
- Sample components
Sample components
-Entire : Agrobacterium phage Milano
| Entire | Name:  Agrobacterium phage Milano (virus) Agrobacterium phage Milano (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Agrobacterium phage Milano
| Supramolecule | Name: Agrobacterium phage Milano / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 2557550 / Sci species name: Agrobacterium phage Milano / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Agrobacterium fabrum str. C58 (bacteria) Agrobacterium fabrum str. C58 (bacteria) |
-Macromolecule #1: Virion-associated protein
| Macromolecule | Name: Virion-associated protein / type: protein_or_peptide / ID: 1 / Number of copies: 18 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Agrobacterium phage Milano (virus) Agrobacterium phage Milano (virus) |
| Molecular weight | Theoretical: 14.673427 KDa |
| Sequence | String: MACNKQNGVK NILITFTDCD TQEVIGPISH EQPDDTLPTY KNCAWTNTAL TNGYVQRSAS NATMTLPVVR DLRVPLAFYQ GCAQVDVQV EKFDGTVMTL TEGAVVEPEE SDGRSVTMNI VASEIDELLP PGSLAAA UniProtKB: Virion-associated protein |
-Macromolecule #2: Tail sheath protein
| Macromolecule | Name: Tail sheath protein / type: protein_or_peptide / ID: 2 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Agrobacterium phage Milano (virus) Agrobacterium phage Milano (virus) |
| Molecular weight | Theoretical: 53.896094 KDa |
| Sequence | String: MAQDALSDGF VRLCIDPSLN FFGEGCKILV EGQMTDDGSA TPDAVTCVTS ELDIIERFGQ GSVLTESLRK VFCTCKSGVS VYALPREDA AAGVKAVYTL TIAGPATTDG RVQLYMGEAE YAVDIGVDAG DTATDIAAAI VAAISPDFPY AATAAAGVIT L TARNAGTI ...String: MAQDALSDGF VRLCIDPSLN FFGEGCKILV EGQMTDDGSA TPDAVTCVTS ELDIIERFGQ GSVLTESLRK VFCTCKSGVS VYALPREDA AAGVKAVYTL TIAGPATTDG RVQLYMGEAE YAVDIGVDAG DTATDIAAAI VAAISPDFPY AATAAAGVIT L TARNAGTI GNHLSVIYTN LGSCTSVTPE GVTVTFAQTT AGSVNPTPND YATVVNECCF AVYVLSSDDT DWQENLRDWI RS AWDCSKP QCFGHGYVFN KGTLGQVLAD GDNSAELSRL ALPTTYPVLP YLTNAAYGAL SACSTCNNPE LNIQGQTFGL LSC INMPES CTPGWTFGEV TQLQANGFVV SGPSTTSGQG NYTSPYIYND VTNYLRDEKN RPNATFRDAS SRRLAAATGV ALAE FLQQF NGLAVFTKNT NIRTGIIGTN PRLMLGKIRK WAQDNVGTLF SEFDNINEDI QLLTDFEVQP KCVGQPGIFH LNMRY RPPV RGARINVNMA PALFDNCDR UniProtKB: Tail sheath protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)