+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human PTH1R in complex with PTH(1-34) and Gs Parathyroid hormone 1 receptor Parathyroid hormone 1 receptor | |||||||||||||||
 Map data Map data | Sharpened consensus map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords |  GPCR / GPCR /  agonist / agonist /  hormone / hormone /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationtype 1 parathyroid hormone receptor binding /  parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / hormone-mediated apoptotic signaling pathway / response to parathyroid hormone / parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / hormone-mediated apoptotic signaling pathway / response to parathyroid hormone /  parathyroid hormone receptor activity / positive regulation of cell proliferation in bone marrow / magnesium ion homeostasis ...type 1 parathyroid hormone receptor binding / parathyroid hormone receptor activity / positive regulation of cell proliferation in bone marrow / magnesium ion homeostasis ...type 1 parathyroid hormone receptor binding /  parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / hormone-mediated apoptotic signaling pathway / response to parathyroid hormone / parathyroid hormone receptor binding / negative regulation of bone mineralization involved in bone maturation / positive regulation of osteoclast proliferation / negative regulation of apoptotic process in bone marrow cell / hormone-mediated apoptotic signaling pathway / response to parathyroid hormone /  parathyroid hormone receptor activity / positive regulation of cell proliferation in bone marrow / magnesium ion homeostasis / positive regulation of signal transduction / macromolecule biosynthetic process / response to fibroblast growth factor / phosphate ion homeostasis / cAMP metabolic process / response to vitamin D / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding / parathyroid hormone receptor activity / positive regulation of cell proliferation in bone marrow / magnesium ion homeostasis / positive regulation of signal transduction / macromolecule biosynthetic process / response to fibroblast growth factor / phosphate ion homeostasis / cAMP metabolic process / response to vitamin D / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding /  bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / PKA activation in glucagon signalling / bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / PKA activation in glucagon signalling /  peptide hormone binding / Rho protein signal transduction / activation of phospholipase C activity / hair follicle placode formation / developmental growth / peptide hormone binding / Rho protein signal transduction / activation of phospholipase C activity / hair follicle placode formation / developmental growth /  D1 dopamine receptor binding / D1 dopamine receptor binding /  intracellular transport / positive regulation of glycogen biosynthetic process / chondrocyte differentiation / Hedgehog 'off' state / positive regulation of bone mineralization / positive regulation of cAMP-mediated signaling / response to cadmium ion / adenylate cyclase-activating adrenergic receptor signaling pathway / homeostasis of number of cells within a tissue / cell maturation / intracellular transport / positive regulation of glycogen biosynthetic process / chondrocyte differentiation / Hedgehog 'off' state / positive regulation of bone mineralization / positive regulation of cAMP-mediated signaling / response to cadmium ion / adenylate cyclase-activating adrenergic receptor signaling pathway / homeostasis of number of cells within a tissue / cell maturation /  bone resorption / activation of adenylate cyclase activity / adenylate cyclase activator activity / trans-Golgi network membrane / bone resorption / activation of adenylate cyclase activity / adenylate cyclase activator activity / trans-Golgi network membrane /  skeletal system development / positive regulation of glucose import / response to lead ion / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / skeletal system development / positive regulation of glucose import / response to lead ion / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / adenylate cyclase-modulating G protein-coupled receptor signaling pathway /  bone development / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / adenylate cyclase-activating G protein-coupled receptor signaling pathway / bone development / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / adenylate cyclase-activating G protein-coupled receptor signaling pathway /  hormone activity / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors / hormone activity / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / Glucagon-type ligand receptors /  platelet aggregation / platelet aggregation /  cognition / Vasopressin regulates renal water homeostasis via Aquaporins / intracellular calcium ion homeostasis / G alpha (z) signalling events / positive regulation of GTPase activity / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / cognition / Vasopressin regulates renal water homeostasis via Aquaporins / intracellular calcium ion homeostasis / G alpha (z) signalling events / positive regulation of GTPase activity / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding /  heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / : / G alpha (12/13) signalling events / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / : / G alpha (12/13) signalling events /  extracellular vesicle / signaling receptor complex adaptor activity / sensory perception of smell / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / cell-cell signaling / extracellular vesicle / signaling receptor complex adaptor activity / sensory perception of smell / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / cell-cell signaling /  GTPase binding GTPase bindingSimilarity search - Function | |||||||||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Lama glama (llama) Lama glama (llama) | |||||||||||||||
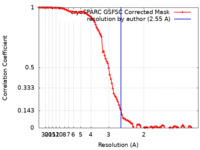
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.55 Å cryo EM / Resolution: 2.55 Å | |||||||||||||||
 Authors Authors | Cary BP / Belousoff MJ / Piper SJ / Wootten D / Sexton PM | |||||||||||||||
| Funding support |  Australia, 4 items Australia, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Structure / Year: 2023 Journal: Structure / Year: 2023Title: Molecular insights into peptide agonist engagement with the PTH receptor. Authors: Brian P Cary / Elliot J Gerrard / Matthew J Belousoff / Madeleine M Fletcher / Yan Jiang / Isabella C Russell / Sarah J Piper / Denise Wootten / Patrick M Sexton /  Abstract: The parathyroid hormone (PTH) 1 receptor (PTH1R) is a G protein-coupled receptor (GPCR) that regulates skeletal development and calcium homeostasis. Here, we describe cryo-EM structures of the PTH1R ...The parathyroid hormone (PTH) 1 receptor (PTH1R) is a G protein-coupled receptor (GPCR) that regulates skeletal development and calcium homeostasis. Here, we describe cryo-EM structures of the PTH1R in complex with fragments of the two hormones, PTH and PTH-related protein, the drug abaloparatide, as well as the engineered tool compounds, long-acting PTH (LA-PTH) and the truncated peptide, M-PTH(1-14). We found that the critical N terminus of each agonist engages the transmembrane bundle in a topologically similar fashion, reflecting similarities in measures of Gαs activation. The full-length peptides induce subtly different extracellular domain (ECD) orientations relative to the transmembrane domain. In the structure bound to M-PTH, the ECD is unresolved, demonstrating that the ECD is highly dynamic when unconstrained by a peptide. High resolutions enabled identification of water molecules near peptide and G protein binding sites. Our results illuminate the action of orthosteric agonists of the PTH1R. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29283.map.gz emd_29283.map.gz | 117.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29283-v30.xml emd-29283-v30.xml emd-29283.xml emd-29283.xml | 29.2 KB 29.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29283_fsc.xml emd_29283_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_29283.png emd_29283.png | 112.8 KB | ||
| Masks |  emd_29283_msk_1.map emd_29283_msk_1.map | 125 MB |  Mask map Mask map | |
| Others |  emd_29283_additional_1.map.gz emd_29283_additional_1.map.gz emd_29283_additional_2.map.gz emd_29283_additional_2.map.gz emd_29283_half_map_1.map.gz emd_29283_half_map_1.map.gz emd_29283_half_map_2.map.gz emd_29283_half_map_2.map.gz | 61.8 MB 60 MB 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29283 http://ftp.pdbj.org/pub/emdb/structures/EMD-29283 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29283 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29283 | HTTPS FTP |
-Related structure data
| Related structure data |  8flqMC  8flrC  8flsC  8fltC  8fluC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29283.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29283.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened consensus map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.65 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_29283_msk_1.map emd_29283_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
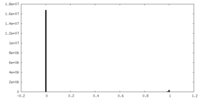
| Density Histograms |
-Additional map: #1
| File | emd_29283_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
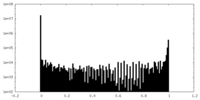
| Density Histograms |
-Additional map: receptor-focused refinement
| File | emd_29283_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | receptor-focused refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
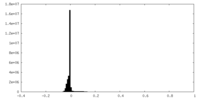
| Density Histograms |
-Half map: Half map 2
| File | emd_29283_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
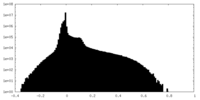
| Density Histograms |
-Half map: Half map 1
| File | emd_29283_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : PTH bound to the PTH1R in complex with G protein
| Entire | Name: PTH bound to the PTH1R in complex with G protein |
|---|---|
| Components |
|
-Supramolecule #1: PTH bound to the PTH1R in complex with G protein
| Supramolecule | Name: PTH bound to the PTH1R in complex with G protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.683434 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE YQLIDCAQYF LDKIDVIKQA DYVPSDQDLL RCRVLTSGIF ETKFQVDKVN FHMFDVGAQR DERRKWIQCF ND VTAIIFV VASSSYNMVI REDNQTNRLQ AALKLFDSIW NNKWLRDTSV ILFLNKQDLL AEKVLAGKSK IEDYFPEFAR YTT PEDATP EPGEDPRVTR AKYFIRDEFL RISTASGDGR HYCYPHFTCA VDTENIRRVF NDCRDIIQRM HLRQYELL |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.413863 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: QSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: QSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.375332 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFR |
-Macromolecule #4: Nanobody35
| Macromolecule | Name: Nanobody35 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Lama glama (llama) Lama glama (llama) |
| Molecular weight | Theoretical: 13.885439 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSS |
-Macromolecule #5: Parathyroid hormone
| Macromolecule | Name: Parathyroid hormone / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 4.125778 KDa |
| Sequence | String: SVSEIQLMHN LGKHLNSMER VEWLRKKLQD VHNF |
-Macromolecule #6: Parathyroid hormone/parathyroid hormone-related peptide receptor
| Macromolecule | Name: Parathyroid hormone/parathyroid hormone-related peptide receptor type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 69.513758 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDLEVLFQG PADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASG KLYPESEEDK EAPTGSRYRG RPCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE L VPGHNRTW ...String: MKTIIALSYI FCLVFADYKD DDDLEVLFQG PADDVMTKEE QIFLLHRAQA QCEKRLKEVL QRPASIMESD KGWTSASTSG KPRKDKASG KLYPESEEDK EAPTGSRYRG RPCLPEWDHI LCWPLGAPGE VVAVPCPDYI YDFNHKGHAY RRCDRNGSWE L VPGHNRTW ANYSECVKFL TNETREREVF DRLGMIYTVG YSVSLASLTV AVLILAYFRR LHCTRNYIHM HLFLSFMLRA VS IFVKDAV LYSGATLDEA ERLTEEELRA IAQAPPPPAT AAAGYAGCRV AVTFFLYFLA TNYYWILVEG LYLHSLIFMA FFS EKKYLW GFTVFGWGLP AVFVAVWVSV RATLANTGCW DLSSGNKKWI IQVPILASIV LNFILFINIV RVLATKLRET NAGR CDTRQ QYRKLLKSTL VLMPLFGVHY IVFMATPYTE VSGTLWQVQM HYEMLFNSFQ GFFVAIIYCF CNGEVQAEIK KSWSR WTLA LDFKRKARSG SSSYSYGPMV SHTSVTNVGP RVGLGLPLSP RLLPTATTNG HPQLPGHAKP GTPALETLET TPPAMA APK DDGFLNGSCS GLDEEASGPE RPPALLQEEW ETVMPAGLEV LFQGPHHHHH HHH |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 14 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.32 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: Buffer also contained 2 micromolar PTH peptide. | |||||||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Details: 20 mA current, negative polarity | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 4750 / Average exposure time: 3.25 sec. / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z
Z Y
Y X
X