[English] 日本語
 Yorodumi
Yorodumi- EMDB-29033: Inactivate state of Maribacter polysiphoniae Argonuate (short pAg... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Inactivate state of Maribacter polysiphoniae Argonuate (short pAgo system) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Argonuate / TIR domain / Oligomerization / NAD+ / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) | |||||||||
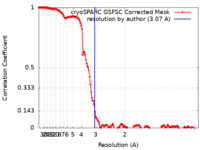
| Method | single particle reconstruction / cryo EM / Resolution: 3.07 Å | |||||||||
 Authors Authors | Shen ZF / Yang XY / Fu TM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Oligomerization-mediated activation of a short prokaryotic Argonaute. Authors: Zhangfei Shen / Xiao-Yuan Yang / Shiyu Xia / Wei Huang / Derek J Taylor / Kotaro Nakanishi / Tian-Min Fu /  Abstract: Although eukaryotic and long prokaryotic Argonaute proteins (pAgos) cleave nucleic acids, some short pAgos lack nuclease activity and hydrolyse NAD(P) to induce bacterial cell death. Here we present ...Although eukaryotic and long prokaryotic Argonaute proteins (pAgos) cleave nucleic acids, some short pAgos lack nuclease activity and hydrolyse NAD(P) to induce bacterial cell death. Here we present a hierarchical activation pathway for SPARTA, a short pAgo consisting of an Argonaute (Ago) protein and TIR-APAZ, an associated protein. SPARTA progresses through distinct oligomeric forms, including a monomeric apo state, a monomeric RNA-DNA-bound state, two dimeric RNA-DNA-bound states and a tetrameric RNA-DNA-bound active state. These snapshots together identify oligomerization as a mechanistic principle of SPARTA activation. The RNA-DNA-binding channel of apo inactive SPARTA is occupied by an auto-inhibitory motif in TIR-APAZ. After the binding of RNA-DNA, SPARTA transitions from a monomer to a symmetric dimer and then an asymmetric dimer, in which two TIR domains interact through charge and shape complementarity. Next, two dimers assemble into a tetramer with a central TIR cluster responsible for hydrolysing NAD(P). In addition, we observe unique features of interactions between SPARTA and RNA-DNA, including competition between the DNA 3' end and the auto-inhibitory motif, interactions between the RNA G2 nucleotide and Ago, and splaying of the RNA-DNA duplex by two loops exclusive to short pAgos. Together, our findings provide a mechanistic basis for the activation of short pAgos, a large section of the Ago superfamily. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29033.map.gz emd_29033.map.gz | 289.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29033-v30.xml emd-29033-v30.xml emd-29033.xml emd-29033.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29033_fsc.xml emd_29033_fsc.xml | 14.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_29033.png emd_29033.png | 65.6 KB | ||
| Others |  emd_29033_additional_1.map.gz emd_29033_additional_1.map.gz emd_29033_half_map_1.map.gz emd_29033_half_map_1.map.gz emd_29033_half_map_2.map.gz emd_29033_half_map_2.map.gz | 263.3 MB 285.3 MB 285.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29033 http://ftp.pdbj.org/pub/emdb/structures/EMD-29033 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29033 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29033 | HTTPS FTP |
-Validation report
| Summary document |  emd_29033_validation.pdf.gz emd_29033_validation.pdf.gz | 1014.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29033_full_validation.pdf.gz emd_29033_full_validation.pdf.gz | 1013.9 KB | Display | |
| Data in XML |  emd_29033_validation.xml.gz emd_29033_validation.xml.gz | 23.4 KB | Display | |
| Data in CIF |  emd_29033_validation.cif.gz emd_29033_validation.cif.gz | 30.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29033 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29033 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29033 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29033 | HTTPS FTP |
-Related structure data
| Related structure data |  8fexMC  8ffiC  8sp0C  8sp3C  8spoC  8squC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29033.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29033.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.5347 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharped map
| File | emd_29033_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharped map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_29033_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_29033_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : MapSPARTA heterodimeric complex(short pAgo)
| Entire | Name: MapSPARTA heterodimeric complex(short pAgo) |
|---|---|
| Components |
|
-Supramolecule #1: MapSPARTA heterodimeric complex(short pAgo)
| Supramolecule | Name: MapSPARTA heterodimeric complex(short pAgo) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
-Macromolecule #1: TIR-APAZ
| Macromolecule | Name: TIR-APAZ / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
| Molecular weight | Theoretical: 53.270594 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRNKIFISHA TPDDNDFTRW LALKLIGLGY EVWCDILFLD KGVDFWSNIE KVIREDTCKF LLVSSSYSNQ REGVLKELAV AAKVKKQLK DDKFIIPLAI DEQLSYDDIN IDIVRLNAID FKMSWARGLK DILEAFEKQK VPKEVADASK SNLLYQQIFL H DKSVIEKE ...String: MRNKIFISHA TPDDNDFTRW LALKLIGLGY EVWCDILFLD KGVDFWSNIE KVIREDTCKF LLVSSSYSNQ REGVLKELAV AAKVKKQLK DDKFIIPLAI DEQLSYDDIN IDIVRLNAID FKMSWARGLK DILEAFEKQK VPKEVADASK SNLLYQQIFL H DKSVIEKE EIYDSNWLSI LSFPEELRFH EYNWMLPKRF DVRELTFPAV RYKNYLCTFA WAYDFTYHLP KTETYHKSKT IR IPTEEIL SGSYDSNFIR NAECKRLIVQ LLNKAFELRM KDKEVQEYEM SNKTAYWLEK GKLEKDKFEK TMLVGKQKDK NWH FAISGA SKLYPFPVLM ISSHIFFTAD GKKLIDSSSV QHSSRRRQGK NWWNNTWRTK LLAFIKYLSD DDTSFYLEMG SEEK VFVSN EPVKFKGNVS YNIPEKNTLE EEAELSGFNQ GEDIEELEEL IENLEAE UniProtKB: TIR domain-containing protein |
-Macromolecule #2: short pAgo
| Macromolecule | Name: short pAgo / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
| Molecular weight | Theoretical: 58.09141 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKELIYIEEP KILFAHGQKC TDARDGLALF GPLNNLYGIK SGVIGTKQGL KIFRDYLDHI QKPIYNSNSI TRPMFPGFEA VFDCKWEST GITFKEVTNE DIGKFLYNSS THKRTYDLVS LFIDKIISAN KNEDENVDVW FVIVPDEIYK YCRPNSVLPK E MVQTKALM ...String: MKELIYIEEP KILFAHGQKC TDARDGLALF GPLNNLYGIK SGVIGTKQGL KIFRDYLDHI QKPIYNSNSI TRPMFPGFEA VFDCKWEST GITFKEVTNE DIGKFLYNSS THKRTYDLVS LFIDKIISAN KNEDENVDVW FVIVPDEIYK YCRPNSVLPK E MVQTKALM SKSKAKSFRY EPSLFPDINI ELKEQEKEAE TYNYDAQFHD QFKARLLKHT IPTQIFREST LAWRDFKNAF GL PIRDFSK IEGHLAWTIS TAAFYKAGGK PWKLSDVRNG VCYLGLVYKK VEKSKNPRNA CCAAQMFLDN GDGTVFKGEV GPW YNPKNG QYHLEPKEAK ALLSQSLQSY KEQIGEYPKE VFIHAKTRFN HQEWDAFLEV TPKETNLVGV TISKTKPLKL YKTE GDYTI LRGNAYVVNE RSAFLWTVGY VPKIQTALSM EVPNPLFIEI NKGEADIKQV LKDILSLTKL NYNACIFADG EPVTL RFAD KIGEILTAST DIKTPPLAFK YYI UniProtKB: Piwi domain-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)