[English] 日本語
 Yorodumi
Yorodumi- EMDB-29021: Structure of dengue virus (DENV2) in complex with prM13, an anti-... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of dengue virus (DENV2) in complex with prM13, an anti-PrM monoclonal antibody | |||||||||
 Map data Map data | ||||||||||
 Sample Sample | Mus musculus != Dengue virus 2 Mus musculus
| |||||||||
 Keywords Keywords | DENV / flavivirus / prM antibody / prM13 / VIRUS-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host JAK-STAT cascade via inhibition of host TYK2 activity / host cell mitochondrion / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT2 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / ribonucleoside triphosphate phosphatase activity / double-stranded RNA binding / channel activity / viral capsid / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell ...symbiont-mediated suppression of host JAK-STAT cascade via inhibition of host TYK2 activity / host cell mitochondrion / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT2 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / ribonucleoside triphosphate phosphatase activity / double-stranded RNA binding / channel activity / viral capsid / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell / mRNA (nucleoside-2'-O-)-methyltransferase activity / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / RNA helicase activity / protein dimerization activity / host cell endoplasmic reticulum membrane / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / induction by virus of host autophagy / viral RNA genome replication / serine-type endopeptidase activity / RNA-dependent RNA polymerase activity / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope / host cell nucleus / virion attachment to host cell / structural molecule activity / virion membrane / proteolysis / extracellular region / ATP binding / membrane / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Dengue virus type 2 / Dengue virus type 2 /   Dengue virus 2 Dengue virus 2 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.8 Å | |||||||||
 Authors Authors | Dowd AD / Sirohi D / Speer S / Mukherjee S / Govero J / Aleshnick M / Larman B / Sukupolvi-Petty S / Sevvana M / Miller AS ...Dowd AD / Sirohi D / Speer S / Mukherjee S / Govero J / Aleshnick M / Larman B / Sukupolvi-Petty S / Sevvana M / Miller AS / Klose T / Zheng A / Kielian M / Kuhn RJ / Diamond MS / Pierson TC | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: prM-reactive antibodies reveal a role for partially mature virions in dengue virus pathogenesis. Authors: Kimberly A Dowd / Devika Sirohi / Scott D Speer / Laura A VanBlargan / Rita E Chen / Swati Mukherjee / Bradley M Whitener / Jennifer Govero / Maya Aleshnick / Bridget Larman / Soila ...Authors: Kimberly A Dowd / Devika Sirohi / Scott D Speer / Laura A VanBlargan / Rita E Chen / Swati Mukherjee / Bradley M Whitener / Jennifer Govero / Maya Aleshnick / Bridget Larman / Soila Sukupolvi-Petty / Madhumati Sevvana / Andrew S Miller / Thomas Klose / Aihua Zheng / Scott Koenig / Margaret Kielian / Richard J Kuhn / Michael S Diamond / Theodore C Pierson /  Abstract: Cleavage of the flavivirus premembrane (prM) structural protein during maturation can be inefficient. The contribution of partially mature flavivirus virions that retain uncleaved prM to pathogenesis ...Cleavage of the flavivirus premembrane (prM) structural protein during maturation can be inefficient. The contribution of partially mature flavivirus virions that retain uncleaved prM to pathogenesis during primary infection is unknown. To investigate this question, we characterized the functional properties of newly-generated dengue virus (DENV) prM-reactive monoclonal antibodies (mAbs) in vitro and using a mouse model of DENV disease. Anti-prM mAbs neutralized DENV infection in a virion maturation state-dependent manner. Alanine scanning mutagenesis and cryoelectron microscopy of anti-prM mAbs in complex with immature DENV defined two modes of attachment to a single antigenic site. In vivo, passive transfer of intact anti-prM mAbs resulted in an antibody-dependent enhancement of disease. However, protection against DENV-induced lethality was observed when the transferred mAbs were genetically modified to inhibit their ability to interact with Fcγ receptors. These data establish that in addition to mature forms of the virus, partially mature infectious prM virions can also contribute to pathogenesis during primary DENV infections. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29021.map.gz emd_29021.map.gz | 202.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29021-v30.xml emd-29021-v30.xml emd-29021.xml emd-29021.xml | 21.6 KB 21.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29021.png emd_29021.png | 231.3 KB | ||
| Filedesc metadata |  emd-29021.cif.gz emd-29021.cif.gz | 7 KB | ||
| Others |  emd_29021_half_map_1.map.gz emd_29021_half_map_1.map.gz emd_29021_half_map_2.map.gz emd_29021_half_map_2.map.gz | 202.3 MB 202.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29021 http://ftp.pdbj.org/pub/emdb/structures/EMD-29021 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29021 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29021 | HTTPS FTP |
-Validation report
| Summary document |  emd_29021_validation.pdf.gz emd_29021_validation.pdf.gz | 856.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29021_full_validation.pdf.gz emd_29021_full_validation.pdf.gz | 855.8 KB | Display | |
| Data in XML |  emd_29021_validation.xml.gz emd_29021_validation.xml.gz | 21.9 KB | Display | |
| Data in CIF |  emd_29021_validation.cif.gz emd_29021_validation.cif.gz | 26.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29021 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29021 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29021 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29021 | HTTPS FTP |
-Related structure data
| Related structure data |  8fe4MC  8fe3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29021.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29021.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.73 Å | ||||||||||||||||||||||||||||||||||||
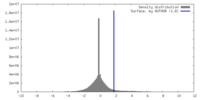
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29021_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
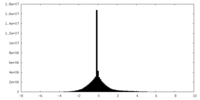
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_29021_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
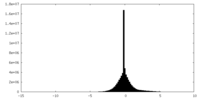
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Mus musculus
| Entire | Name:  |
|---|---|
| Components |
|
-Supramolecule #1: Dengue virus 2
| Supramolecule | Name: Dengue virus 2 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 11060 / Sci species name: Dengue virus 2 / Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Virus shell | Shell ID: 1 / Diameter: 500.0 Å / T number (triangulation number): 3 |
-Macromolecule #1: Envelope protein E
| Macromolecule | Name: Envelope protein E / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Dengue virus type 2 Dengue virus type 2 |
| Molecular weight | Theoretical: 43.892469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRCIGMSNRD FVEGVSGGSW VDIVLEHGSC VTTMAKNKPT LDFELIKTEA KQPATLRKYC IEAKLTNTTT ESRCPTQGEP SLNEEQDKR FVCKHSMVDR GWGNGCGLFG KGGIVTCAMF RCKKNMEGKV VQPENLEYTI VITPHSGEEH AVGNDTGKHG K EIKITPQS ...String: MRCIGMSNRD FVEGVSGGSW VDIVLEHGSC VTTMAKNKPT LDFELIKTEA KQPATLRKYC IEAKLTNTTT ESRCPTQGEP SLNEEQDKR FVCKHSMVDR GWGNGCGLFG KGGIVTCAMF RCKKNMEGKV VQPENLEYTI VITPHSGEEH AVGNDTGKHG K EIKITPQS SITEAELTGY GTVTMECSPR TGLDFNEMVL LQMENKAWLV HRQWFLDLPL PWLPGADTQG SNWIQKETLV TF KNPHAKK QDVVVLGSQE GAMHTALTGA TEIQMSSGNL LFTGHLKCRL RMDKLQLKGM SYSMCTGKFK VVKEIAETQH GTI VIRVQY EGDGSPCKIP FEIMDLEKRH VLGRLITVNP IVTEKDSPVN IEAEPPFGDS YIIIGVEPGQ LKLNWFKK UniProtKB: Genome polyprotein |
-Macromolecule #2: prM protein
| Macromolecule | Name: prM protein / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Dengue virus type 2 Dengue virus type 2 |
| Molecular weight | Theoretical: 9.261531 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: FHLTTRNGEP HMIVSRQEKG KSLLFKTEDG VNMCTLMAMD LGELCEDTIT YKCPLLRQNE PEDIDCWCNS TSTWVTYGTC T UniProtKB: Genome polyprotein |
-Macromolecule #3: prM13 Fab Heavy Chain
| Macromolecule | Name: prM13 Fab Heavy Chain / type: protein_or_peptide / ID: 3 Details: The homology model of the prM13 variable heavy chain domain (1-118) was pieced together with the crystal structure of the constant heavy chain domain, derived from ZV-67, which belongs to ...Details: The homology model of the prM13 variable heavy chain domain (1-118) was pieced together with the crystal structure of the constant heavy chain domain, derived from ZV-67, which belongs to murine IgG2c isotype similar to prM13. Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.777656 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AAQLQESGPE LVKPGASVKI SCKASGYTFT DYFINWVRQS HGKSLEWIGD FYPNNRGPTY NQKFEGKATL TVDKSSSTAY MELRSLTSD DSAVYYCARG LWDAWLSYWG QGTLVTVSAA KTTAPSVYPL APVCGGTTGS SVTLGCLVKG YFPEPVTLTW N SGSLSSGV ...String: AAQLQESGPE LVKPGASVKI SCKASGYTFT DYFINWVRQS HGKSLEWIGD FYPNNRGPTY NQKFEGKATL TVDKSSSTAY MELRSLTSD DSAVYYCARG LWDAWLSYWG QGTLVTVSAA KTTAPSVYPL APVCGGTTGS SVTLGCLVKG YFPEPVTLTW N SGSLSSGV HTFPALLQSG LYTLSSSVTV TSNTWPSQTI TCNVAHPASS TKVDKKIEPR VPI |
-Macromolecule #4: prM13 Fab Light Chain
| Macromolecule | Name: prM13 Fab Light Chain / type: protein_or_peptide / ID: 4 Details: The homology model of the prM13 variable light chain domain (1-106) was pieced together with the crystal structure of the constant light chain domain, derived from ZV-67, which belongs to ...Details: The homology model of the prM13 variable light chain domain (1-106) was pieced together with the crystal structure of the constant light chain domain, derived from ZV-67, which belongs to murine IgG2c isotype similar to prM13. Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.24551 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AIVATQSPAI MSASPGEKVT ISCSASSSVS YMYWYQQKPG SSPKPWIYRT SNLASGVPTR FSGSGSGTSY SFTISSMEAE DAATYYCQQ YHNYPRTFGG GTKAKSARAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK ...String: AIVATQSPAI MSASPGEKVT ISCSASSSVS YMYWYQQKPG SSPKPWIYRT SNLASGVPTR FSGSGSGTSY SFTISSMEAE DAATYYCQQ YHNYPRTFGG GTKAKSARAD AAPTVSIFPP SSEQLTSGGA SVVCFLNNFY PKDINVKWKI DGSERQNGVL N SWTDQDSK DSTYSMSSTL TLTKDEYERH NSYTCEATHK TSTSPIVKSF NRNEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: PELCO Ultrathin Carbon with Lacey Carbon / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: LACEY |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Resolution.type: BY AUTHOR / Resolution: 9.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: jspr / Number images used: 5458 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: PROJECTION MATCHING |
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||
| Output model |  PDB-8fe4: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)