[English] 日本語
 Yorodumi
Yorodumi- EMDB-28084: Structure of Lates calcarifer DNA polymerase theta polymerase dom... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Lates calcarifer DNA polymerase theta polymerase domain with hairpin DNA | |||||||||
 Map data Map data | Structure of Lates calcarifer DNA polymerase theta polymerase domain with hairpin DNA | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA double-strand break repair / Microhomology-mediated end joining / DNA BINDING PROTEIN / DNA BINDING PROTEIN-DNA complex | |||||||||
| Biological species |  Lates calcarifer (barramundi perch) Lates calcarifer (barramundi perch) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Li C / Zhu H / Sun J / Gao Y | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2023 Journal: Nucleic Acids Res / Year: 2023Title: Structural basis of DNA polymerase θ mediated DNA end joining. Authors: Chuxuan Li / Hanwen Zhu / Shikai Jin / Leora M Maksoud / Nikhil Jain / Ji Sun / Yang Gao /  Abstract: DNA polymerase θ (Pol θ) plays an essential role in the microhomology-mediated end joining (MMEJ) pathway for repairing DNA double-strand breaks. However, the mechanisms by which Pol θ recognizes ...DNA polymerase θ (Pol θ) plays an essential role in the microhomology-mediated end joining (MMEJ) pathway for repairing DNA double-strand breaks. However, the mechanisms by which Pol θ recognizes microhomologous DNA ends and performs low-fidelity DNA synthesis remain unclear. Here, we present cryo-electron microscope structures of the polymerase domain of Lates calcarifer Pol θ with long and short duplex DNA at up to 2.4 Å resolution. Interestingly, Pol θ binds to long and short DNA substrates similarly, with extensive interactions around the active site. Moreover, Pol θ shares a similar active site as high-fidelity A-family polymerases with its finger domain well-closed but differs in having hydrophilic residues surrounding the nascent base pair. Computational simulations and mutagenesis studies suggest that the unique insertion loops of Pol θ help to stabilize short DNA binding and assemble the active site for MMEJ repair. Taken together, our results illustrate the structural basis of Pol θ-mediated MMEJ. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28084.map.gz emd_28084.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28084-v30.xml emd-28084-v30.xml emd-28084.xml emd-28084.xml | 16.3 KB 16.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28084.png emd_28084.png | 87.4 KB | ||
| Filedesc metadata |  emd-28084.cif.gz emd-28084.cif.gz | 6.1 KB | ||
| Others |  emd_28084_half_map_1.map.gz emd_28084_half_map_1.map.gz emd_28084_half_map_2.map.gz emd_28084_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28084 http://ftp.pdbj.org/pub/emdb/structures/EMD-28084 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28084 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28084 | HTTPS FTP |
-Validation report
| Summary document |  emd_28084_validation.pdf.gz emd_28084_validation.pdf.gz | 835.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28084_full_validation.pdf.gz emd_28084_full_validation.pdf.gz | 834.8 KB | Display | |
| Data in XML |  emd_28084_validation.xml.gz emd_28084_validation.xml.gz | 12.4 KB | Display | |
| Data in CIF |  emd_28084_validation.cif.gz emd_28084_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28084 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28084 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28084 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28084 | HTTPS FTP |
-Related structure data
| Related structure data |  8efkMC  8ef9C  8efcC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28084.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28084.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of Lates calcarifer DNA polymerase theta polymerase domain with hairpin DNA | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||
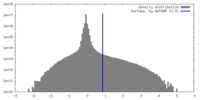
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Structure of Lates calcarifer DNA polymerase theta polymerase...
| File | emd_28084_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of Lates calcarifer DNA polymerase theta polymerase domain with hairpin DNA | ||||||||||||
| Projections & Slices |
| ||||||||||||
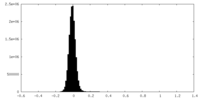
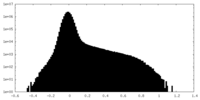
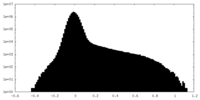
| Density Histograms |
-Half map: Structure of Lates calcarifer DNA polymerase theta polymerase...
| File | emd_28084_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of Lates calcarifer DNA polymerase theta polymerase domain with hairpin DNA | ||||||||||||
| Projections & Slices |
| ||||||||||||
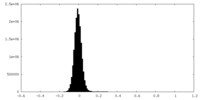
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of Lates calcarifer DNA polymerase Theta with dup...
| Entire | Name: Ternary complex of Lates calcarifer DNA polymerase Theta with duplex DNA and incoming nucleotide |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of Lates calcarifer DNA polymerase Theta with dup...
| Supramolecule | Name: Ternary complex of Lates calcarifer DNA polymerase Theta with duplex DNA and incoming nucleotide type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Lates calcarifer (barramundi perch) Lates calcarifer (barramundi perch) |
-Macromolecule #1: Lates calcarifer DNA polymerase theta
| Macromolecule | Name: Lates calcarifer DNA polymerase theta / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Lates calcarifer (barramundi perch) Lates calcarifer (barramundi perch) |
| Molecular weight | Theoretical: 95.674117 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGHHHHHHPS GVKTENNDHI NLKVAGQDGS VVQFKIKRHT PLSKLMKAYC ERQGLSMRQI RFRFDGQPIN ETDTPAQLEM EDEDTIDVF QQQTGGCMDP PSDAESPVTD DGFTLQLSQD ASLCPSNSGT FSIIDVASDR RLFNTFIKEW KTKERYSLAL A CEKREHIQ ...String: MGHHHHHHPS GVKTENNDHI NLKVAGQDGS VVQFKIKRHT PLSKLMKAYC ERQGLSMRQI RFRFDGQPIN ETDTPAQLEM EDEDTIDVF QQQTGGCMDP PSDAESPVTD DGFTLQLSQD ASLCPSNSGT FSIIDVASDR RLFNTFIKEW KTKERYSLAL A CEKREHIQ QPEGEIGGKH KRAPAARQKL NRTDGFPVRD SDGLVLIGLS VCWGARDSYY ISLQQEQSKG LSSSLAPPPL DD DLPVSER LGQVRSCLSR PSAGLRGGVV VTYDIIQVYK TLVLSCGISL AGNCEDPKVA CWLLDPGSEE RTLPNMVTVY CPE ELPLLD GLGSAHAHCP RVRAATKSVL VHAVMNHLTG LLEKDSMLDL FRSIEMPSQV CLALLELNGV GFSVEECERQ KHVM QAKLT ALESQAYNLA GHSFSLTSID DIAQVLFLEL HLPPNGDVGG SKSKKTLGYT RRGGGRVRLG KQFSTTKDIL EKLRP LHPL PGVILEWRRI TNALTKVVFP LQREKQYHPT LAMDRIYPIA QTHTATGRVS FTEPNIQNVP KDFEICMPTV VGESPP SQN GCQMTTKPGK NRRSVAPSVT GGAAEQGPAF SVSMRHAFVP FSGGMILAAD YSQLELRVLA HLSKDQRLLQ VLNGGAD VF RCIAAEWKGV DPETVNDSLR QQAKQICYGI IYGMGAKSLG EQMGVEENDA ACYIESFKAR YKGINAFLKE TVKNCIKN G YVQTLMGRRR YLPGISNTNT HIKAHAERQA VNTTVQGSAA DIVKLATVNI QKRLRKTYPT APLSHQHTHS GTSQYRAGT SHLRGAFFVL QLHDELIYET REEDLIQVAQ IVKREMESAV KLYVKLKAKV KVGPSWGNLQ DLDL |
-Macromolecule #2: DNA (5'-D(P*TP*TP*TP*TP*GP*GP*CP*TP*TP*TP*TP*GP*CP*CP*(2DA))-3')
| Macromolecule | Name: DNA (5'-D(P*TP*TP*TP*TP*GP*GP*CP*TP*TP*TP*TP*GP*CP*CP*(2DA))-3') type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Lates calcarifer (barramundi perch) Lates calcarifer (barramundi perch) |
| Molecular weight | Theoretical: 7.278689 KDa |
| Sequence | String: (DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DG)(DG) (DC)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT)(DT) (DG)(DC)(DC)(2DA) |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #4: 2',3'-dideoxyadenosine triphosphate
| Macromolecule | Name: 2',3'-dideoxyadenosine triphosphate / type: ligand / ID: 4 / Number of copies: 1 / Formula: DDS |
|---|---|
| Molecular weight | Theoretical: 475.182 Da |
| Chemical component information | 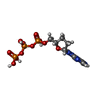 ChemComp-DDS: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL |
|---|---|
| Buffer | pH: 7.6 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 288920 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)