+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | P. gingivalis RNA Polymerase | |||||||||
 Map data Map data | P. gingivalis RNA polymerase | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Porphyromonas gingivalis / RNA polymerase / transcription / CFB group bacteria | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA-directed RNA polymerase complex / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase / protein dimerization activity / DNA-templated transcription / magnesium ion binding / DNA binding / zinc ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Porphyromonas gingivalis (bacteria) Porphyromonas gingivalis (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Liu B / Bu F | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2024 Journal: J Mol Biol / Year: 2024Title: Cryo-EM Structure of Porphyromonas gingivalis RNA Polymerase. Authors: Fan Bu / Xiaoxuan Wang / Mengke Li / Li Ma / Chuan Wang / Yangbo Hu / Zhengguo Cao / Bin Liu /   Abstract: Porphyromonas gingivalis, an anaerobic CFB (Cytophaga, Fusobacterium, and Bacteroides) group bacterium, is the keystone pathogen of periodontitis and has been implicated in various systemic diseases. ...Porphyromonas gingivalis, an anaerobic CFB (Cytophaga, Fusobacterium, and Bacteroides) group bacterium, is the keystone pathogen of periodontitis and has been implicated in various systemic diseases. Increased antibiotic resistance and lack of effective antibiotics necessitate a search for new intervention strategies. Here we report a 3.5 Å resolution cryo-EM structure of P. gingivalis RNA polymerase (RNAP). The structure displays new structural features in its ω subunit and multiple domains in β and β' subunits, which differ from their counterparts in other bacterial RNAPs. Superimpositions with E. coli RNAP holoenzyme and initiation complex further suggest that its ω subunit may contact the σ4 domain, thereby possibly contributing to the assembly and stabilization of initiation complexes. In addition to revealing the unique features of P. gingivalis RNAP, our work offers a framework for future studies of transcription regulation in this important pathogen, as well as for structure-based drug development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27487.map.gz emd_27487.map.gz | 64.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27487-v30.xml emd-27487-v30.xml emd-27487.xml emd-27487.xml | 24.6 KB 24.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27487.png emd_27487.png | 102.8 KB | ||
| Filedesc metadata |  emd-27487.cif.gz emd-27487.cif.gz | 7.8 KB | ||
| Others |  emd_27487_additional_1.map.gz emd_27487_additional_1.map.gz emd_27487_half_map_1.map.gz emd_27487_half_map_1.map.gz emd_27487_half_map_2.map.gz emd_27487_half_map_2.map.gz | 66.7 MB 120.6 MB 120.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27487 http://ftp.pdbj.org/pub/emdb/structures/EMD-27487 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27487 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27487 | HTTPS FTP |
-Validation report
| Summary document |  emd_27487_validation.pdf.gz emd_27487_validation.pdf.gz | 792.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27487_full_validation.pdf.gz emd_27487_full_validation.pdf.gz | 792.2 KB | Display | |
| Data in XML |  emd_27487_validation.xml.gz emd_27487_validation.xml.gz | 14.2 KB | Display | |
| Data in CIF |  emd_27487_validation.cif.gz emd_27487_validation.cif.gz | 16.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27487 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27487 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27487 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27487 | HTTPS FTP |
-Related structure data
| Related structure data |  8dkcMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27487.map.gz / Format: CCP4 / Size: 129.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27487.map.gz / Format: CCP4 / Size: 129.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | P. gingivalis RNA polymerase | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.89 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: P. gingivalis RNA polymerase-sharpened map
| File | emd_27487_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | P. gingivalis RNA polymerase-sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
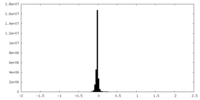
| Density Histograms |
-Half map: half map A
| File | emd_27487_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map_A | ||||||||||||
| Projections & Slices |
| ||||||||||||
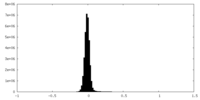
| Density Histograms |
-Half map: half map B
| File | emd_27487_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map_B | ||||||||||||
| Projections & Slices |
| ||||||||||||
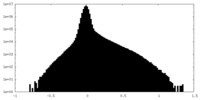
| Density Histograms |
- Sample components
Sample components
-Entire : Porphyromonas gingivalis RNA polymerase
| Entire | Name: Porphyromonas gingivalis RNA polymerase |
|---|---|
| Components |
|
-Supramolecule #1: Porphyromonas gingivalis RNA polymerase
| Supramolecule | Name: Porphyromonas gingivalis RNA polymerase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (bacteria) Porphyromonas gingivalis (bacteria) |
| Molecular weight | Theoretical: 389 KDa |
-Macromolecule #1: DNA-directed RNA polymerase subunit alpha
| Macromolecule | Name: DNA-directed RNA polymerase subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: DNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (bacteria) / Strain: ATCC BAA-308 / W83 Porphyromonas gingivalis (bacteria) / Strain: ATCC BAA-308 / W83 |
| Molecular weight | Theoretical: 36.838078 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAILAFQKPE NVLMMETSDS IAKFEFKPLE PGYGITIGNA LRRILLSSLE GFAITAIKIE GVEHEFATIP GVLEDVTNII LNLKQVRFK QIVPNADVEK ATIVISNSEV FRAGDLNAQL SNFEVLNSNL VICHLDKSAT LTMEFSINKG RGYVSAEENR A EHNELSTI ...String: MAILAFQKPE NVLMMETSDS IAKFEFKPLE PGYGITIGNA LRRILLSSLE GFAITAIKIE GVEHEFATIP GVLEDVTNII LNLKQVRFK QIVPNADVEK ATIVISNSEV FRAGDLNAQL SNFEVLNSNL VICHLDKSAT LTMEFSINKG RGYVSAEENR A EHNELSTI AIDSIYTPIR NVKYAVENFR VEQKTDYEKL LMEVTTDGSI RPVDALREAA QILISHFSLF AENKIAIEYV DI VDTDEFD EDSLHMRQLL KSKLSGLDLS VRALNCLNAA GVDTLGDLVS LSRSDLMKIR NFGKKSLTEL DELLATLNLS FGM DISKYK LDKD UniProtKB: DNA-directed RNA polymerase subunit alpha |
-Macromolecule #2: DNA-directed RNA polymerase subunit beta
| Macromolecule | Name: DNA-directed RNA polymerase subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (bacteria) Porphyromonas gingivalis (bacteria) |
| Molecular weight | Theoretical: 142.524562 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTPTTNNKRI NFASIKNPLF YPDFLEVQLK SFHDFLQLDT PPERRKKEGL YKVFAENFPI TDTRNNFVLE FLDYYIDPPK YSIEECLSR GLTYSVPLKA KLKLYCTDPD HEDFATVIQD VFLGPIPYMT SSGTFVINGA ERVIVSQLHR SPGVFFGQSL H TNGTKLYS ...String: MTPTTNNKRI NFASIKNPLF YPDFLEVQLK SFHDFLQLDT PPERRKKEGL YKVFAENFPI TDTRNNFVLE FLDYYIDPPK YSIEECLSR GLTYSVPLKA KLKLYCTDPD HEDFATVIQD VFLGPIPYMT SSGTFVINGA ERVIVSQLHR SPGVFFGQSL H TNGTKLYS ARIIPFKGSW IEFATDINNV MYAYIDRKKK LPVTTLLRAI GFEADKDILD IFNLADEVKV TKANLKKCIG RK LAARVIN TYIDDLSDED TGEVVSMERI TVVVDREVEL TEDNIEAILN SNAQTILLHR NDSNTSDYSI IFNTLQKDPC NSE KEALYY VYRQLRNAEP ADDASAREVI TNLFFSDKRY DLGDVGRYRI NKKLNLNIDP DIKVLTNEDI IEIIKYLIEL VNSK ASVDD IDHLSNRRVR TVGEQLYNQF GIGLARMART VRDRMNVRDN EVFTPIDLVN AKTISSVVNS FFGTNALSQF MDQTN PLAE ITHKRRLSAL GPGGLSRERA GFEVRDVHYT HYGRLCPIET PEGPNIGLIS SLCVYAKISD LGFITTPYRE VKNGKV DFS DNGLKYYTAE EEEEKTVAQG NAPLDENGRF VRERVKARYE SDFPLVTPDE VDLMDVSPTQ IASIAAALIP FLEHDDA NR ALMGSNMMRQ AVPLLRPESP IVGTGIEGKL VKDSRTQIVA ERGGEVVFVD ASCIKIRYDR TADEEFVSFD DAIVTYYL P KYRKTNQSTT IDLHPICSKG DRVEAGQILT EGYSTQGGEL ALGRNVQVAY MPWKGYNYED AIVLNERMVR EDFFTSVHV DEYILEVRET KRGLEELTSD IPNVSEDATR DLDENGIVRI GAHIEPGDIL IGKITPKGES DPTPEEKLLR AIFGDKAGDV KDASLKATP SLRGVVIDTK LFSKAAKKKS RTSTKEAVSK LDETYAKRQQ QLHERLIEKL TELTKGKTCC GVKDYLNVEL I KAGSKFTK KDLEALDFNV IQLSDWTNDA HTNELIKAVA VNYLKHSKEI EAELRRRKLD ETIGDELPAG IVQMAKVYIA KK RKIQVGD KMAGRHGNKG IVSKIVRQED MPFLADGTPV DICLNPLGVP SRMNLGQIFE AVLAWAGRKM NVKFATPIFD GAS LNDMNE WTDKAGLPRD GKTYLYDGGT GERFDQPATV GVTYFLKLGH MVDDKMHARS IGPYSLITQQ PLGGKAQFGG QRFG EMEVW ALEAFGASHI LQEILTVKSD DVVGRSKAYE AIVKGDPMPT PGIPESLNVL LHELKGLGLS FSLD UniProtKB: DNA-directed RNA polymerase subunit beta |
-Macromolecule #3: DNA-directed RNA polymerase subunit beta'
| Macromolecule | Name: DNA-directed RNA polymerase subunit beta' / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (bacteria) Porphyromonas gingivalis (bacteria) |
| Molecular weight | Theoretical: 161.311781 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAFRKENKIK NNFSKIRITL ASPEEILENS FGEVLKPETI NYRTYKPERD GLFCERIFGP VKDFECHCGK YKRIRYRGIV CDRCGVEVT EKKVRRERMG HIHLVVPVAH IWYFRSLPNK IGYLLGLPTK KLDAIIYYER YVVIQPGVAE GLSQLDLLSE E EYLDKLDE ...String: MAFRKENKIK NNFSKIRITL ASPEEILENS FGEVLKPETI NYRTYKPERD GLFCERIFGP VKDFECHCGK YKRIRYRGIV CDRCGVEVT EKKVRRERMG HIHLVVPVAH IWYFRSLPNK IGYLLGLPTK KLDAIIYYER YVVIQPGVAE GLSQLDLLSE E EYLDKLDE IERTHKGNQN LEDTNPDKFI AKIGAEAIYD LLCRVDLDSI SYELRDRANT DGSQQRKTEA LKRLQVVESF RA SKGVNRP EWMVMKVIPV IPPDLRPLVP LDGGRFATSD LNDLYRRVII RNNRLKRLIE IKAPEVILRN EKRMLQEAVD SLF DNSRKS SAVKSDNNRP LKSLSDSLKG KQGRFRQNLL GKRVDYSARS VIVVGPELKM HECGLPKDMA AELYKPFIIR KLIE RGIVK TVKSAKKIVD RKEPVIWDIL EYVMKGHPVL LNRAPTLHRL GIQAFQPKLI EGKAIQLHPL SCTAFNADFD GDQMA VHLP LSNEAILEAQ LLMLASHNIL NPANGAPITV PSQDMVLGLY YITKLRPNTK GHGLIFYGPE EATIAYNEGK VDIHAP IKV YVEDYENGEL VRRMVETSVG RLMVNEYVPK KVGYVNEVLG KKALRDIIGS VIKICGVATT AKFLDDIKNL GYYMAFK GG LSFNLADVLI PDEKDQLIQE GYTAVEQIMQ DYSMGFITFN ERYNQIIDTW THINGRLSNV LIKQLSSDND GFNSVFMM M DSGARGSKEQ IRQLSGMRGL MAKPQKSGAE GGQIIENPIL SNFKEGLSVL EYFISTHGAR KGLADTALKT ADAGYLTRR LVDVSHDVII TEEDCGTLRG LLTTELKQNE DVVASLYERI LGRVSVHDII HPTTGDIIVR AGEEIREQAA QIIEDSPIEA VEIRSVLTC ESKKGVCAKC YGRNLATNRM VQRGEVVGVI AAQSIGEPGT QLTLRTFHVG GIASNVATEN SLLSKYDGIL E FEELRAVD ATDESHQVVV SRMTELRIAD PNTGIILANH NIPYGAKLFF RQGDAVKKGD KIIEWDPFNA VIVSEVAGTL SF EGVVENV TFKMESDETT GLKEKIIIES KDKTMAPYAR IIDENGEMLK NYSLPMGAHV VKDDGDTVKV GEILVKIPRS VGK AGDITG GLPRVTELFE ARNPSNPAIV SEIDGEIGFG KLKRGNREIT VTSKLGEEKK YLIPLSKQLL VQENDFVRAG TPLS DGAIT PADILAIKGP TAVQEYIVNE VQDVYRLQGV KINDKHFEVI VRQMMRKVEI VDPGDTLFLE QQVVDKFEVM EENDR IWGK KVVIDAGDSQ VLKAGQIVTA RKLRDENSML KRKDLKIVKV RDAKSATASQ ILQGITRAAL QTKSFMSAAS FQETTK VLN EAAICGKTDY LEGLKENVIC GHLIPAGTGL RDYEKLVVMH RDDYEKATAE RKSFLSEPTA EPAMEEAPSE HHHHHH UniProtKB: DNA-directed RNA polymerase subunit beta' |
-Macromolecule #4: RNA polymerase Rpb6
| Macromolecule | Name: RNA polymerase Rpb6 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (bacteria) Porphyromonas gingivalis (bacteria) |
| Molecular weight | Theoretical: 12.932515 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MELKKMNVPM DTITRDMVRL SEDTENVYET VMIIAKRANQ IGQQMKQDLE KKLQDFSSSN DNLEEVFENR EQIEISRYYE HLPKPGLIA TAEYEQDKLY HRMPGATSTN D UniProtKB: DNA-directed RNA polymerase subunit omega |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 20 mM Tris-HCl, pH 8.0, 50 mM NaCl, 5 mM DTT, 8mM CHAPSO |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: NITROGEN |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Average exposure time: 32.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 2.15) / Number images used: 137270 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 2.15) |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 2.15) |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Correlation coefficient |
|---|---|
| Output model |  PDB-8dkc: |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)