+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Human liver ferritin | |||||||||
 マップデータ マップデータ | ||||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | human / liver / ferritin / OXIDOREDUCTASE | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報iron ion sequestering activity / : / autolysosome / Scavenging by Class A Receptors / Golgi Associated Vesicle Biogenesis / ferroxidase / intracellular sequestering of iron ion / ferroxidase activity / negative regulation of fibroblast proliferation / ferric iron binding ...iron ion sequestering activity / : / autolysosome / Scavenging by Class A Receptors / Golgi Associated Vesicle Biogenesis / ferroxidase / intracellular sequestering of iron ion / ferroxidase activity / negative regulation of fibroblast proliferation / ferric iron binding / Iron uptake and transport / ferrous iron binding / tertiary granule lumen / iron ion transport / intracellular iron ion homeostasis / ficolin-1-rich granule lumen / iron ion binding / immune response / negative regulation of cell population proliferation / Neutrophil degranulation / extracellular exosome / extracellular region / identical protein binding / membrane / nucleus / cytoplasm / cytosol 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
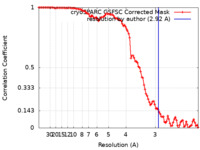
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 2.92 Å | |||||||||
 データ登録者 データ登録者 | Zhang Z | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: To Be Published ジャーナル: To Be Publishedタイトル: Human liver ferritin 著者: Zhang Z | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_27437.map.gz emd_27437.map.gz | 97.1 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-27437-v30.xml emd-27437-v30.xml emd-27437.xml emd-27437.xml | 14.4 KB 14.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_27437_fsc.xml emd_27437_fsc.xml | 9.8 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_27437.png emd_27437.png | 85.9 KB | ||
| Filedesc metadata |  emd-27437.cif.gz emd-27437.cif.gz | 4.8 KB | ||
| その他 |  emd_27437_additional_1.map.gz emd_27437_additional_1.map.gz emd_27437_half_map_1.map.gz emd_27437_half_map_1.map.gz emd_27437_half_map_2.map.gz emd_27437_half_map_2.map.gz | 51.8 MB 95.2 MB 95.2 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27437 http://ftp.pdbj.org/pub/emdb/structures/EMD-27437 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27437 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27437 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_27437_validation.pdf.gz emd_27437_validation.pdf.gz | 1.1 MB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_27437_full_validation.pdf.gz emd_27437_full_validation.pdf.gz | 1.1 MB | 表示 | |
| XML形式データ |  emd_27437_validation.xml.gz emd_27437_validation.xml.gz | 18.4 KB | 表示 | |
| CIF形式データ |  emd_27437_validation.cif.gz emd_27437_validation.cif.gz | 23.6 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27437 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27437 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27437 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27437 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8dhxMC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_27437.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_27437.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ボクセルのサイズ | X=Y=Z: 1.08 Å | ||||||||||||||||||||
| 密度 |
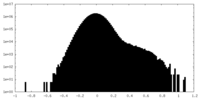
| ||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-追加マップ: #1
| ファイル | emd_27437_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
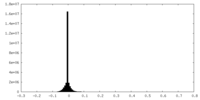
| 密度ヒストグラム |
-ハーフマップ: #2
| ファイル | emd_27437_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
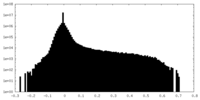
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_27437_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
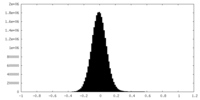
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : human liver ferritin
| 全体 | 名称: human liver ferritin |
|---|---|
| 要素 |
|
-超分子 #1: human liver ferritin
| 超分子 | 名称: human liver ferritin / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Ferritin heavy chain
| 分子 | 名称: Ferritin heavy chain / タイプ: protein_or_peptide / ID: 1 / コピー数: 24 / 光学異性体: LEVO / EC番号: ferroxidase |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 21.255656 KDa |
| 配列 | 文字列: MTTASTSQVR QNYHQDSEAA INRQINLELY ASYVYLSMSY YFDRDDVALK NFAKYFLHQS HEEREHAEKL MKLQNQRGGR IFLQDIKKP DCDDWESGLN AMECALHLEK NVNQSLLELH KLATDKNDPH LCDFIETHYL NEQVKAIKEL GDHVTNLRKM G APESGLAE YLFDKHTLGD SDNES UniProtKB: Ferritin heavy chain |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| グリッド | モデル: Quantifoil R1.2/1.3 / 前処理 - タイプ: GLOW DISCHARGE |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 平均電子線量: 41.25 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 3.591 µm / 最小 デフォーカス(公称値): 0.544 µm / 倍率(公称値): 82000 |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー








 Z
Z Y
Y X
X