+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
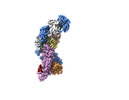
| Title | type I-C Cascade bound to AcrIF2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host CRISPR-cas system / maintenance of CRISPR repeat elements /  endonuclease activity / defense response to virus / endonuclease activity / defense response to virus /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  RNA binding RNA bindingSimilarity search - Function | |||||||||
| Biological species |   Desulfovibrio vulgaris (bacteria) / Desulfovibrio vulgaris (bacteria) /  Casadabanvirus D3112 / Casadabanvirus D3112 /   Desulfovibrio vulgaris str. Hildenborough (bacteria) Desulfovibrio vulgaris str. Hildenborough (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | O'Brien RE / Bravo JPK / Ramos D / Hibshman GN / Wright JT / Taylor DW | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Structural snapshots of R-loop formation by a type I-C CRISPR Cascade. Authors: Roisin E O'Brien / Jack P K Bravo / Delisa Ramos / Grace N Hibshman / Jacquelyn T Wright / David W Taylor /  Abstract: Type I CRISPR-Cas systems employ multi-subunit Cascade effector complexes to target foreign nucleic acids for destruction. Here, we present structures of D. vulgaris type I-C Cascade at various ...Type I CRISPR-Cas systems employ multi-subunit Cascade effector complexes to target foreign nucleic acids for destruction. Here, we present structures of D. vulgaris type I-C Cascade at various stages of double-stranded (ds)DNA target capture, revealing mechanisms that underpin PAM recognition and Cascade allosteric activation. We uncover an interesting mechanism of non-target strand (NTS) DNA stabilization via stacking interactions with the "belly" subunits, securing the NTS in place. This "molecular seatbelt" mechanism facilitates efficient R-loop formation and prevents dsDNA reannealing. Additionally, we provide structural insights into how two anti-CRISPR (Acr) proteins utilize distinct strategies to achieve a shared mechanism of type I-C Cascade inhibition by blocking PAM scanning. These observations form a structural basis for directional R-loop formation and reveal how different Acr proteins have converged upon common molecular mechanisms to efficiently shut down CRISPR immunity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27412.map.gz emd_27412.map.gz | 267.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27412-v30.xml emd-27412-v30.xml emd-27412.xml emd-27412.xml | 21.2 KB 21.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27412.png emd_27412.png | 38.5 KB | ||
| Others |  emd_27412_half_map_1.map.gz emd_27412_half_map_1.map.gz emd_27412_half_map_2.map.gz emd_27412_half_map_2.map.gz | 262.4 MB 262.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27412 http://ftp.pdbj.org/pub/emdb/structures/EMD-27412 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27412 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27412 | HTTPS FTP |
-Related structure data
| Related structure data |  8dfsMC  8dejC  8dexC  8dfaC  8dfoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27412.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27412.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.94 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_27412_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_27412_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : type I-C Cascade bound to AcrIF2
| Entire | Name: type I-C Cascade bound to AcrIF2 |
|---|---|
| Components |
|
-Supramolecule #1: type I-C Cascade bound to AcrIF2
| Supramolecule | Name: type I-C Cascade bound to AcrIF2 / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#6 |
|---|
-Supramolecule #2: type I-C Cascade
| Supramolecule | Name: type I-C Cascade / type: complex / ID: 2 / Chimera: Yes / Parent: 1 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:   Desulfovibrio vulgaris (bacteria) / Strain: Hildenborough Desulfovibrio vulgaris (bacteria) / Strain: Hildenborough |
-Supramolecule #3: AcrIF2
| Supramolecule | Name: AcrIF2 / type: complex / ID: 3 / Chimera: Yes / Parent: 1 / Macromolecule list: #6 |
|---|---|
| Source (natural) | Organism:  Casadabanvirus D3112 Casadabanvirus D3112 |
-Macromolecule #1: pre-crRNA processing endonuclease
| Macromolecule | Name: pre-crRNA processing endonuclease / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number:  Hydrolases; Acting on ester bonds Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:   Desulfovibrio vulgaris (bacteria) Desulfovibrio vulgaris (bacteria)Strain: ATCC 29579 / DSM 644 / NCIMB 8303 / VKM B-1760 / Hildenborough |
| Molecular weight | Theoretical: 25.977857 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MTHGAVKTYG IRLRVWGDYA CFTRPEMKVE RVSYDVMPPS AARGILEAIH WKPAIRWIVD RIHVLRPIVF DNVRRNEVSS KIPKPNPAT AMRDRKPLYF LVDDGSNRQQ RAATLLRNVD YVIEAHFELT DKAGAEDNAG KHLDIFRRRA RAGQSFQQPC L GCREFPAS ...String: MTHGAVKTYG IRLRVWGDYA CFTRPEMKVE RVSYDVMPPS AARGILEAIH WKPAIRWIVD RIHVLRPIVF DNVRRNEVSS KIPKPNPAT AMRDRKPLYF LVDDGSNRQQ RAATLLRNVD YVIEAHFELT DKAGAEDNAG KHLDIFRRRA RAGQSFQQPC L GCREFPAS FELLEGDVPL SCYAGEKRDL GYMLLDIDFE RDMTPLFFKA VMEDGVITPP SRTSPEVRA |
-Macromolecule #2: CRISPR-associated protein, TM1801 family
| Macromolecule | Name: CRISPR-associated protein, TM1801 family / type: protein_or_peptide / ID: 2 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Desulfovibrio vulgaris (bacteria) / Strain: Hildenborough / ATCC 29579 / DSM 644 / NCIMB 8303 Desulfovibrio vulgaris (bacteria) / Strain: Hildenborough / ATCC 29579 / DSM 644 / NCIMB 8303 |
| Molecular weight | Theoretical: 32.358912 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MTAIANRYEF VLLFDVENGN PNGDPDAGNM PRIDPETGHG LVTDVCLKRK IRNHVALTKE GAERFNIYIQ EKAILNETHE RAYTACDLK PEPKKLPKKV EDAKRVTDWM CTNFYDIRTF GAVMTTEVNC GQVRGPVQMA FARSVEPVVP QEVSITRMAV T TKAEAEKQ ...String: MTAIANRYEF VLLFDVENGN PNGDPDAGNM PRIDPETGHG LVTDVCLKRK IRNHVALTKE GAERFNIYIQ EKAILNETHE RAYTACDLK PEPKKLPKKV EDAKRVTDWM CTNFYDIRTF GAVMTTEVNC GQVRGPVQMA FARSVEPVVP QEVSITRMAV T TKAEAEKQ QGDNRTMGRK HIVPYGLYVA HGFISAPLAE KTGFSDEDLT LFWDALVNMF EHDRSAARGL MSSRKLIVFK HQ NRLGNAP AHKLFDLVKV SRAEGSSGPA RSFADYAVTV GQAPEGVEVK EML |
-Macromolecule #3: CRISPR-associated protein, CT1133 family
| Macromolecule | Name: CRISPR-associated protein, CT1133 family / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Desulfovibrio vulgaris str. Hildenborough (bacteria) Desulfovibrio vulgaris str. Hildenborough (bacteria)Strain: ATCC 29579 / DSM 644 / NCIMB 8303 / VKM B-1760 / Hildenborough |
| Molecular weight | Theoretical: 68.123219 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MILQALHGYY QRMSADPDAG MPPYGTSMEN ISFALVLDAK GTLRGIEDLR EQEGKKLRPR KMLVPIAEKK GNGIKPNFLW ENTSYILGV DAKGKQERTD KCHAAFIAHI KAYCDTADQD LAAVLQFLEH GEKDLSAFPV SEEVIGSNIV FRIEGEPGFV H ERPAARQA ...String: MILQALHGYY QRMSADPDAG MPPYGTSMEN ISFALVLDAK GTLRGIEDLR EQEGKKLRPR KMLVPIAEKK GNGIKPNFLW ENTSYILGV DAKGKQERTD KCHAAFIAHI KAYCDTADQD LAAVLQFLEH GEKDLSAFPV SEEVIGSNIV FRIEGEPGFV H ERPAARQA WANCLNRREQ GLCGQCLITG ERQKPIAQLH PSIKGGRDGV RGAQAVASIV SFNNTAFESY GKEQSINAPV SQ EAAFSYV TALNYLLNPS NRQKVTIADA TVVFWAERSS PAEDIFAGMF DPPSTTAKPE SSNGTPPEDS EEGSQPDTAR DDP HAAARM HDLLVAIRSG KRATDIMPDM DESVRFHVLG LSPNAARLSV RFWEVDTVGH MLDKVGRHYR ELEIIPQFNN EQEF PSLST LLRQTAVLNK TENISPVLAG GLFRAMLTGG PYPQSLLPAV LGRIRAEHAR PEDKSRYRLE VVTYYRAALI KAYLI RNRK LEVPVSLDPA RTDRPYLLGR LFAVLEKAQE DAVPGANATI KDRYLASASA NPGQVFHMLL KNASNHTAKL RKDPER KGS AIHYEIMMQE IIDNISDFPV TMSSDEQGLF MIGYYHQRKA LFTKKNKEN |
-Macromolecule #4: CRISPR-associated protein, CT1133 family
| Macromolecule | Name: CRISPR-associated protein, CT1133 family / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Desulfovibrio vulgaris str. Hildenborough (bacteria) Desulfovibrio vulgaris str. Hildenborough (bacteria)Strain: Hildenborough / ATCC 29579 / DSM 644 / NCIMB 8303 |
| Molecular weight | Theoretical: 14.017981 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: VSLDPARTDR PYLLGRLFAV LEKAQEDAVP GANATIKDRY LASASANPGQ VFHMLLKNAS NHTAKLRKDP ERKGSAIHYE IMMQEIIDN ISDFPVTMSS DEQGLFMIGY YHQRKALFTK KNKEN |
-Macromolecule #6: Anti-CRISPR protein 30
| Macromolecule | Name: Anti-CRISPR protein 30 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Casadabanvirus D3112 Casadabanvirus D3112 |
| Molecular weight | Theoretical: 9.9316 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MIAQQHKDTV AACEAAEAIA IAKDQVWDGE GYTKYTFDDN SVLIQSGTTQ YAMDADDADS IKGYADWLDD EARSAEASEI ERLLESVEE E |
-Macromolecule #5: RNA (48-MER)
| Macromolecule | Name: RNA (48-MER) / type: rna / ID: 5 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:   Desulfovibrio vulgaris (bacteria) / Strain: Hildenborough Desulfovibrio vulgaris (bacteria) / Strain: Hildenborough |
| Molecular weight | Theoretical: 15.476264 KDa |
| Sequence | String: GGAUUGAAAC GCCAUGCUCA GGCUGGCGAG UGCGCGCCAC UCAUCAAG |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.5 e/Å2 |
- Image processing
Image processing
| Initial angle assignment | Type: OTHER |
|---|---|
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 21625 |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X