[English] 日本語
 Yorodumi
Yorodumi- EMDB-27329: Cryo-EM structure of cyanopindolol-bound beta1-adrenergic recepto... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of cyanopindolol-bound beta1-adrenergic receptor in complex with heterotrimeric Gs-protein | |||||||||
 Map data Map data | composite map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationOlfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / sensory perception of chemical stimulus / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / Activation of the phototransduction cascade / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma ...Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / sensory perception of chemical stimulus / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / Activation of the phototransduction cascade / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Glucagon-type ligand receptors / G alpha (12/13) signalling events / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / beta-2 adrenergic receptor binding / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Thrombin signalling through proteinase activated receptors (PARs) / Ca2+ pathway / Extra-nuclear estrogen signaling / G alpha (z) signalling events / G alpha (s) signalling events / G alpha (q) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (i) signalling events / Vasopressin regulates renal water homeostasis via Aquaporins / D1 dopamine receptor binding / adenylate cyclase-activating adrenergic receptor signaling pathway / viral release from host cell by cytolysis / ionotropic glutamate receptor binding / insulin-like growth factor receptor binding / adenylate cyclase activator activity / peptidoglycan catabolic process / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / photoreceptor disc membrane / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / G-protein beta-subunit binding / heterotrimeric G-protein complex / cell wall macromolecule catabolic process / signaling receptor complex adaptor activity / lysozyme / lysozyme activity / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / host cell cytoplasm / defense response to bacterium / G protein-coupled receptor signaling pathway / GTPase activity / GTP binding / membrane / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |    | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Su M / Paknejad N / Hite RK / Huang XY | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structures of β-adrenergic receptor in complex with Gs and ligands of different efficacies. Authors: Minfei Su / Navid Paknejad / Lan Zhu / Jinan Wang / Hung Nguyen Do / Yinglong Miao / Wei Liu / Richard K Hite / Xin-Yun Huang /  Abstract: G-protein-coupled receptors (GPCRs) receive signals from ligands with different efficacies, and transduce to heterotrimeric G-proteins to generate different degrees of physiological responses. ...G-protein-coupled receptors (GPCRs) receive signals from ligands with different efficacies, and transduce to heterotrimeric G-proteins to generate different degrees of physiological responses. Previous studies revealed how ligands with different efficacies activate GPCRs. Here, we investigate how a GPCR activates G-proteins upon binding ligands with different efficacies. We report the cryo-EM structures of β-adrenergic receptor (β-AR) in complex with Gs (GαGβGγ) and a partial agonist or a very weak partial agonist, and compare them to the β-AR-Gs structure in complex with a full agonist. Analyses reveal similar overall complex architecture, with local conformational differences. Cellular functional studies with mutations of β-AR residues show effects on the cellular signaling from β-AR to the cAMP response initiated by the three different ligands, with residue-specific functional differences. Biochemical investigations uncover that the intermediate state complex comprising β-AR and nucleotide-free Gs is more stable when binding a full agonist than a partial agonist. Molecular dynamics simulations support the local conformational flexibilities and different stabilities among the three complexes. These data provide insights into the ligand efficacy in the activation of GPCRs and G-proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27329.map.gz emd_27329.map.gz | 202 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27329-v30.xml emd-27329-v30.xml emd-27329.xml emd-27329.xml | 25.8 KB 25.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27329.png emd_27329.png | 75.3 KB | ||
| Others |  emd_27329_additional_1.map.gz emd_27329_additional_1.map.gz emd_27329_additional_2.map.gz emd_27329_additional_2.map.gz emd_27329_additional_3.map.gz emd_27329_additional_3.map.gz emd_27329_half_map_1.map.gz emd_27329_half_map_1.map.gz emd_27329_half_map_2.map.gz emd_27329_half_map_2.map.gz | 59.7 MB 59.7 MB 59.7 MB 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27329 http://ftp.pdbj.org/pub/emdb/structures/EMD-27329 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27329 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27329 | HTTPS FTP |
-Validation report
| Summary document |  emd_27329_validation.pdf.gz emd_27329_validation.pdf.gz | 676.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27329_full_validation.pdf.gz emd_27329_full_validation.pdf.gz | 676.1 KB | Display | |
| Data in XML |  emd_27329_validation.xml.gz emd_27329_validation.xml.gz | 14.1 KB | Display | |
| Data in CIF |  emd_27329_validation.cif.gz emd_27329_validation.cif.gz | 16 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27329 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27329 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27329 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27329 | HTTPS FTP |
-Related structure data
| Related structure data |  8dcsMC  8dcrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27329.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27329.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | composite map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.7093 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: consensus map
| File | emd_27329_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | consensus map | ||||||||||||
| Projections & Slices |
| ||||||||||||
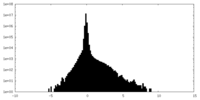
| Density Histograms |
-Additional map: G-protein focused map
| File | emd_27329_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | G-protein focused map | ||||||||||||
| Projections & Slices |
| ||||||||||||
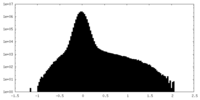
| Density Histograms |
-Additional map: Receptor focused map
| File | emd_27329_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Receptor focused map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: consensus map-half A
| File | emd_27329_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | consensus_map-half_A | ||||||||||||
| Projections & Slices |
| ||||||||||||
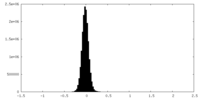
| Density Histograms |
-Half map: consensus map-half B
| File | emd_27329_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | consensus_map-half_B | ||||||||||||
| Projections & Slices |
| ||||||||||||
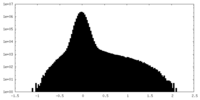
| Density Histograms |
- Sample components
Sample components
-Entire : cyanopindolol-bound beta1-adrenergic receptor in complex with het...
| Entire | Name: cyanopindolol-bound beta1-adrenergic receptor in complex with heterotrimeric Gs-protein |
|---|---|
| Components |
|
-Supramolecule #1: cyanopindolol-bound beta1-adrenergic receptor in complex with het...
| Supramolecule | Name: cyanopindolol-bound beta1-adrenergic receptor in complex with heterotrimeric Gs-protein type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 37.285734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW ...String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NAICFFPNGN AF ATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGHDNRVSCL GVT DDGMAV ATGSWDSFLK IWN |
-Macromolecule #2: Nanobody 35
| Macromolecule | Name: Nanobody 35 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.282897 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AAQVQLQESG GGLVQPGGSL RLSCAASGFT FSNYKMNWVR QAPGKGLEWV SDISQSGASI SYTGSVKGRF TISRDNAKNT LYLQMNSLK PEDTAVYYCA RCPAPFTRDC FDVTSTTYAY RGQGTQVTVS SHHHHHHEPE A |
-Macromolecule #3: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 44.694551 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPLAMGCLGN SKTEDQRNEE KGQREANKKI EKQLQKDKQV YRATHRLLLL GAGESGKSTI VKQMRILHVN GFNGDSEKAT KVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV RACYERSNEY Q LIDCAQYF ...String: GPLAMGCLGN SKTEDQRNEE KGQREANKKI EKQLQKDKQV YRATHRLLLL GAGESGKSTI VKQMRILHVN GFNGDSEKAT KVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV RACYERSNEY Q LIDCAQYF LDKIDVIKQD DYVPSDQDLL RCRVLTSGIF ETKFQVDKVN FHMFDVGGQR DERRKWIQCF NDVTAIIFVV AS SSYNMVI REDNQTNRLQ EALNLFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK IEDYFPEFAR YTTPEDATPE PGE DPRVTR AKYFIRDEFL RISTASGDGR HYCYPHFTCA VDTENIRRVF NDCRDIIQRM HLRQYELL |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.845078 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFSAI L |
-Macromolecule #5: Endolysin,Endolysin,Beta-1 adrenergic receptor chimera
| Macromolecule | Name: Endolysin,Endolysin,Beta-1 adrenergic receptor chimera type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO / EC number: lysozyme |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 57.958668 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKLEVLFQ GPNIFEMLRI DEGLRLKIYK DTEGYYTIGI GHLLTKSPSL NAAKSELDKA IGRNTNGVI TKDEAEKLFN QDVDAAVRGI LRNAKLKPVY DSLDAVRRAA LINMVFQMGE TGVAGFTNSL RMLQQKRWDE A AVNLAKSR ...String: MKTIIALSYI FCLVFADYKD DDDKLEVLFQ GPNIFEMLRI DEGLRLKIYK DTEGYYTIGI GHLLTKSPSL NAAKSELDKA IGRNTNGVI TKDEAEKLFN QDVDAAVRGI LRNAKLKPVY DSLDAVRRAA LINMVFQMGE TGVAGFTNSL RMLQQKRWDE A AVNLAKSR WYNQTPNRAK RVITTFRTGT WDAYAAGAEL LSQQWEAGMS LLMALVVLLI VAGNVLVIAA IGSTQRLQTL TN LFITSLA CADLVVGLLV VPFGATLVVR GTWLWGSFLC ELWTSLDVLC VTASIETLCV IAIDRYLAIT SPFRYQSLMT RAR AKVIIC TVWAISALVS FLPIMMHWWR DEDPQALKCY QDPGCCDFVT NRAYAIASSI ISFYIPLLIM IFVYLRVYRE AKEQ IRKID RCEGRFREHK ALKTLGIIMG VFTLCWLPFF LVNIVNVFNR DLVPDWLFVF FNWLGYANSA FNPIIYCRSP DFRKA FKRL LCFPRKADRR LEVLFQGPHH HHHH |
-Macromolecule #6: 4-{[(2S)-3-(tert-butylamino)-2-hydroxypropyl]oxy}-3H-indole-2-car...
| Macromolecule | Name: 4-{[(2S)-3-(tert-butylamino)-2-hydroxypropyl]oxy}-3H-indole-2-carbonitrile type: ligand / ID: 6 / Number of copies: 1 / Formula: P32 |
|---|---|
| Molecular weight | Theoretical: 287.357 Da |
| Chemical component information | 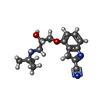 ChemComp-P32: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.8 mg/mL |
|---|---|
| Buffer | pH: 7 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 28.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 657613 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)