+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | SthK closed state, cAMP-bound in the presence of detergent | ||||||||||||
 Map data Map data | Cyclic nucleotide-gate ion channel SthK in detergent (DDM) in the presence of ligand (cAMP) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | cyclic nucleotide-gated channel / lipid modulation / pacemaker channel / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationintracellular cyclic nucleotide activated cation channel complex / intracellularly cGMP-activated cation channel activity / intracellularly cAMP-activated cation channel activity / cGMP binding / protein-containing complex binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) / Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) /   Spirochaeta thermophila DSM 6578 (bacteria) Spirochaeta thermophila DSM 6578 (bacteria) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | ||||||||||||
 Authors Authors | Rheinberger J / Schmidpeter PA | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Anionic lipids unlock the gates of select ion channels in the pacemaker family. Authors: Philipp A M Schmidpeter / Di Wu / Jan Rheinberger / Paul M Riegelhaupt / Haiping Tang / Carol V Robinson / Crina M Nimigean /    Abstract: Lipids play important roles in regulating membrane protein function, but the molecular mechanisms used are elusive. Here we investigated how anionic lipids modulate SthK, a bacterial pacemaker ...Lipids play important roles in regulating membrane protein function, but the molecular mechanisms used are elusive. Here we investigated how anionic lipids modulate SthK, a bacterial pacemaker channel homolog, and HCN2, whose activity contributes to pacemaking in the heart and brain. Using SthK allowed the reconstitution of purified channels in controlled lipid compositions for functional and structural assays that are not available for the eukaryotic channels. We identified anionic lipids bound tightly to SthK and their exact binding locations and determined that they potentiate channel activity. Cryo-EM structures in the most potentiating lipids revealed an open state and identified a nonannular lipid bound with its headgroup near an intersubunit salt bridge that clamps the intracellular channel gate shut. Breaking this conserved salt bridge abolished lipid modulation in SthK and eukaryotic HCN2 channels, indicating that anionic membrane lipids facilitate channel opening by destabilizing these interactions. Our findings underline the importance of state-dependent protein-lipid interactions. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25981.map.gz emd_25981.map.gz | 59.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25981-v30.xml emd-25981-v30.xml emd-25981.xml emd-25981.xml | 23.4 KB 23.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25981_fsc.xml emd_25981_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_25981.png emd_25981.png | 67.6 KB | ||
| Masks |  emd_25981_msk_1.map emd_25981_msk_1.map emd_25981_msk_2.map emd_25981_msk_2.map | 64 MB 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-25981.cif.gz emd-25981.cif.gz | 7.1 KB | ||
| Others |  emd_25981_additional_1.map.gz emd_25981_additional_1.map.gz emd_25981_half_map_1.map.gz emd_25981_half_map_1.map.gz emd_25981_half_map_2.map.gz emd_25981_half_map_2.map.gz | 59 MB 45.7 MB 45.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25981 http://ftp.pdbj.org/pub/emdb/structures/EMD-25981 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25981 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25981 | HTTPS FTP |
-Validation report
| Summary document |  emd_25981_validation.pdf.gz emd_25981_validation.pdf.gz | 996.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25981_full_validation.pdf.gz emd_25981_full_validation.pdf.gz | 996 KB | Display | |
| Data in XML |  emd_25981_validation.xml.gz emd_25981_validation.xml.gz | 16 KB | Display | |
| Data in CIF |  emd_25981_validation.cif.gz emd_25981_validation.cif.gz | 21.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25981 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25981 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25981 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25981 | HTTPS FTP |
-Related structure data
| Related structure data |  7tktMC  7tj5C  7tj6C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25981.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25981.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cyclic nucleotide-gate ion channel SthK in detergent (DDM) in the presence of ligand (cAMP) | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0961 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
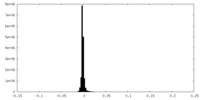
-Mask #1
| File |  emd_25981_msk_1.map emd_25981_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
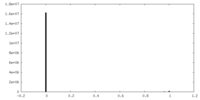
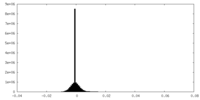
| Density Histograms |
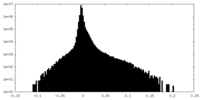
-Mask #2
| File |  emd_25981_msk_2.map emd_25981_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
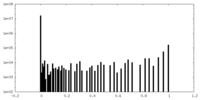
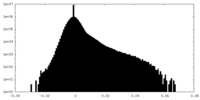
| Density Histograms |
-Additional map: Postprocessed map for SthK after refinement focused on...
| File | emd_25981_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map for SthK after refinement focused on transmembrane domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
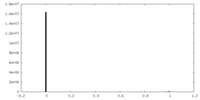
| Density Histograms |
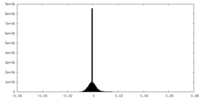
-Half map: Unfiltered half map 1 of the full length...
| File | emd_25981_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half map 1 of the full length SthK, used in refinement and gold standard FSC resolution calculation | ||||||||||||
| Projections & Slices |
| ||||||||||||
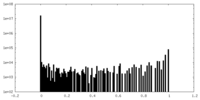
| Density Histograms |
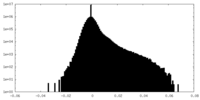
-Half map: Unfiltered half map 2 of the full length...
| File | emd_25981_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered half map 2 of the full length SthK, used in refinement and gold standard FSC resolution calculation | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : closed conformation of SthK bound to cAMP
| Entire | Name: closed conformation of SthK bound to cAMP |
|---|---|
| Components |
|
-Supramolecule #1: closed conformation of SthK bound to cAMP
| Supramolecule | Name: closed conformation of SthK bound to cAMP / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: SthK was purified in dodecyl maltoside (DDM). |
|---|---|
| Source (natural) | Organism:   Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) Spirochaeta thermophila (strain ATCC 700085 / DSM 6578 / Z-1203) (bacteria) |
-Macromolecule #1: Putative transcriptional regulator, Crp/Fnr family
| Macromolecule | Name: Putative transcriptional regulator, Crp/Fnr family / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Spirochaeta thermophila DSM 6578 (bacteria) / Strain: ATCC 700085 / DSM 6578 / Z-1203 Spirochaeta thermophila DSM 6578 (bacteria) / Strain: ATCC 700085 / DSM 6578 / Z-1203 |
| Molecular weight | Theoretical: 51.118574 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAKDIGINSD PNSSSVDKLM KSSGVSNPTY TLVWKVWILA VTLYYAIRIP LTLVFPSLFS PLLPLDILAS LALIADIPLD LAFESRRTS GRKPTLLAPS RLPDLLAALP LDLLVFALHL PSPLSLLSLV RLLKLISVQR SATRILSYRI NPALLRLLSL V GFILLAAH ...String: MAKDIGINSD PNSSSVDKLM KSSGVSNPTY TLVWKVWILA VTLYYAIRIP LTLVFPSLFS PLLPLDILAS LALIADIPLD LAFESRRTS GRKPTLLAPS RLPDLLAALP LDLLVFALHL PSPLSLLSLV RLLKLISVQR SATRILSYRI NPALLRLLSL V GFILLAAH GIACGWMSLQ PPSENPAGTR YLSAFYWTIT TLTTIGYGDI TPSTPTQTVY TIVIELLGAA MYGLVIGNIA SL VSKLDAA KLLHRERVER VTAFLSYKRI SPELQRRIIE YFDYLWETRR GYEEREVLKE LPHPLRLAVA MEIHGDVIEK VPL FKGAGE EFIRDIILHL EPVIYGPGEY IIRAGEMGSD VYFINRGSVE VLSADEKTRY AILSEGQFFG EMALILRAPR TATV RARAF CDLYRLDKET FDRILSRYPE IAAQIQELAV RRKELESSGL VPRGSVKHHH H UniProtKB: Transcriptional regulator, Crp/Fnr family |
-Macromolecule #2: ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE
| Macromolecule | Name: ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: CMP |
|---|---|
| Molecular weight | Theoretical: 329.206 Da |
| Chemical component information |  ChemComp-CMP: |
-Macromolecule #3: (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]o...
| Macromolecule | Name: (1R)-2-{[(S)-{[(2S)-2,3-dihydroxypropyl]oxy}(hydroxy)phosphoryl]oxy}-1-[(hexadecanoyloxy)methyl]ethyl (9Z)-octadec-9-enoate type: ligand / ID: 3 / Number of copies: 52 / Formula: PGW |
|---|---|
| Molecular weight | Theoretical: 749.007 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295.15 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||||||||
| Details | WT SthK in dodecyl maltoside (DDM) |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Spherical aberration corrector: TFS Cs corrector / Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Number real images: 2561 / Average exposure time: 8.0 sec. / Average electron dose: 55.77 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated magnification: 45616 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.001 mm / Nominal defocus max: 2.75 µm / Nominal defocus min: 1.25 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z
Z Y
Y X
X