[English] 日本語
 Yorodumi
Yorodumi- EMDB-25308: Tertiary structure of an individual particle of self-folding RNA ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Tertiary structure of an individual particle of self-folding RNA polymer (particle #073) | ||||||||||||||||||
 Map data Map data | Tertiary structure of an individual particle of self-folding RNA polymer (particle #073) | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Single molecule structure / Individual Particle cryo-Electron Tomography / IPET / Cryo-ET / tertiary structure / Structural Flexibility / Self-folding mechanism / RNA origami / RNA | ||||||||||||||||||
| Biological species | synthetic construct (others) | ||||||||||||||||||
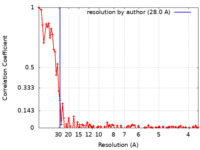
| Method | electron tomography / cryo EM / Resolution: 28.0 Å | ||||||||||||||||||
 Authors Authors | Liu J / Ren G | ||||||||||||||||||
| Funding support |  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Non-averaged single-molecule tertiary structures reveal RNA self-folding through individual-particle cryo-electron tomography. Authors: Jianfang Liu / Ewan K S McRae / Meng Zhang / Cody Geary / Ebbe Sloth Andersen / Gang Ren /    Abstract: Large-scale and continuous conformational changes in the RNA self-folding process present significant challenges for structural studies, often requiring trade-offs between resolution and ...Large-scale and continuous conformational changes in the RNA self-folding process present significant challenges for structural studies, often requiring trade-offs between resolution and observational scope. Here, we utilize individual-particle cryo-electron tomography (IPET) to examine the post-transcriptional self-folding process of designed RNA origami 6-helix bundle with a clasp helix (6HBC). By avoiding selection, classification, averaging, or chemical fixation and optimizing cryo-ET data acquisition parameters, we reconstruct 120 three-dimensional (3D) density maps from 120 individual particles at an electron dose of no more than 168 eÅ, achieving averaged resolutions ranging from 23 to 35 Å, as estimated by Fourier shell correlation (FSC) at 0.5. Each map allows us to identify distinct RNA helices and determine a unique tertiary structure. Statistical analysis of these 120 structures confirms two reported conformations and reveals a range of kinetically trapped, intermediate, and highly compacted states, demonstrating a maturation folding landscape likely driven by helix-helix compaction interactions. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25308.map.gz emd_25308.map.gz | 6.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25308-v30.xml emd-25308-v30.xml emd-25308.xml emd-25308.xml | 12.5 KB 12.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25308_fsc.xml emd_25308_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_25308.png emd_25308.png | 91.6 KB | ||
| Filedesc metadata |  emd-25308.cif.gz emd-25308.cif.gz | 5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25308 http://ftp.pdbj.org/pub/emdb/structures/EMD-25308 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25308 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25308 | HTTPS FTP |
-Validation report
| Summary document |  emd_25308_validation.pdf.gz emd_25308_validation.pdf.gz | 305.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25308_full_validation.pdf.gz emd_25308_full_validation.pdf.gz | 305.1 KB | Display | |
| Data in XML |  emd_25308_validation.xml.gz emd_25308_validation.xml.gz | 10.3 KB | Display | |
| Data in CIF |  emd_25308_validation.cif.gz emd_25308_validation.cif.gz | 13.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25308 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25308 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25308 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25308 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_25308.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25308.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Tertiary structure of an individual particle of self-folding RNA polymer (particle #073) | ||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.88 Å | ||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : RNA Origami 6 Helix Bundle with Clasp (6HBC)
| Entire | Name: RNA Origami 6 Helix Bundle with Clasp (6HBC) |
|---|---|
| Components |
|
-Supramolecule #1: RNA Origami 6 Helix Bundle with Clasp (6HBC)
| Supramolecule | Name: RNA Origami 6 Helix Bundle with Clasp (6HBC) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: The RNA was transcribed from linearized DNA template using T7 RNA polymerase purified in house. |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 230 KDa |
-Macromolecule #1: RNA Origami 6 Helix Bundle with Clasp (6HBC)
| Macromolecule | Name: RNA Origami 6 Helix Bundle with Clasp (6HBC) / type: rna / ID: 1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Sequence | String: GGGAGAGUAC UAUUCAGAUG CAGACCGCAA GUUCAGAGCG GUUUGCAUCU AGGGUACGUU UUCGAACGUA UCCUCCGACU AAGUGUAUUC GUAUACUUAG UGCCUUGUGC CUGCUUCGGC AGGCAUGACC CAAAUGUGCC UUUCGGGGCA CAUUUCCGGU CAUCCAAGUU ...String: GGGAGAGUAC UAUUCAGAUG CAGACCGCAA GUUCAGAGCG GUUUGCAUCU AGGGUACGUU UUCGAACGUA UCCUCCGACU AAGUGUAUUC GUAUACUUAG UGCCUUGUGC CUGCUUCGGC AGGCAUGACC CAAAUGUGCC UUUCGGGGCA CAUUUCCGGU CAUCCAAGUU CGCUUGGGUG AUGCGGGCGU AUAGGUUCGU CUAUACGUCC GCGUUUUCCG AGAAGAGGUA ACUCGGGAAA CCGGUCCACG UGACAAAGGU AGAGUUACGU GGAGGGAGCA GCUGCAAAGG GAUAAUGCAG UUGCUGGCUG GAUGCCAGAA CUCACGACUG GCAUCUACGG GGAUGGUGCU CUCCCAAUUC UCCAUUUACC GCCGAAUCGA CCCCAACGUG AGAGGGGUCG GUUCCCCGAG CAUAGACCAA UAUCCCAGGU UUAUGCUCCC CAACGCUGGA CGAACUACCU ACGUCUAGCG UUCCGGCAAA UGAGUCAAUA CCUCAGACUU AUUUGCGGUG CCUGAGCCUA AACUGAACAU GGGUUCAGGC AUCUUGGCUC CAGUUCGCUG GAGCCGACGG UAGCGCUGCG UUCGCGCAGU GCUAGGGAGC AUCCGUUUUC GAGCGGAUGC UGGGCGGUUG CCUGUUCGCA GGCAAUCGGG CCUACUCAUG AUUCGUCAUG AGUGGUGACA GCGUGAUGUU CGCAUUACGC UGUCGGGUAG AUGGAGAAUU |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron tomography |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: LEICA EM GP | ||||||||||||
| Details | In vitro transcribed RNA was purified by size exclusion chromatography and spin concentrated in amicon spin columns. | ||||||||||||
| Sectioning | Other: NO SECTIONING |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 21 / Average exposure time: 0.88 sec. / Average electron dose: 8.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












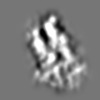




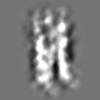




















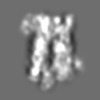
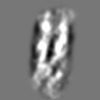




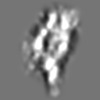
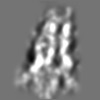





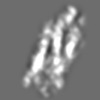






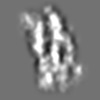


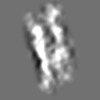
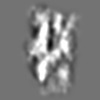


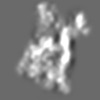

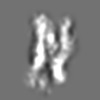
















































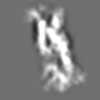



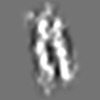


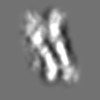

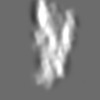



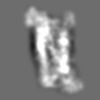
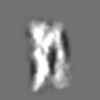





















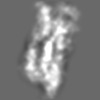


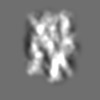





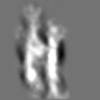



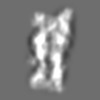




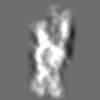

 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)