+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-20383 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | CryoEM structure of zebra fish alpha-1 glycine receptor bound with Taurine in SMA, desensitized state | |||||||||
 マップデータ マップデータ | CryoEM structure of zebra fish alpha-1 glycine receptor bound with Taurine in SMA, desensitized state | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | glycine receptor / SMA / CryoEM / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報Neurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / cellular response to ethanol / transmitter-gated monoatomic ion channel activity / cellular response to zinc ion / regulation of neuron differentiation / neurotransmitter receptor activity / glycine binding / transmembrane transporter complex ...Neurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / cellular response to ethanol / transmitter-gated monoatomic ion channel activity / cellular response to zinc ion / regulation of neuron differentiation / neurotransmitter receptor activity / glycine binding / transmembrane transporter complex / chloride channel complex / ligand-gated monoatomic ion channel activity / response to amino acid / monoatomic ion transport / chloride transmembrane transport / central nervous system development / cellular response to amino acid stimulus / transmembrane signaling receptor activity / perikaryon / postsynaptic membrane / neuron projection / dendrite / synapse / zinc ion binding / membrane / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.0 Å | |||||||||
 データ登録者 データ登録者 | Yu J / Zhu H | |||||||||
| 資金援助 |  米国, 1件 米国, 1件
| |||||||||
 引用 引用 |  ジャーナル: Cell / 年: 2021 ジャーナル: Cell / 年: 2021タイトル: Mechanism of gating and partial agonist action in the glycine receptor. 著者: Jie Yu / Hongtao Zhu / Remigijus Lape / Timo Greiner / Juan Du / Wei Lü / Lucia Sivilotti / Eric Gouaux /   要旨: Ligand-gated ion channels mediate signal transduction at chemical synapses and transition between resting, open, and desensitized states in response to neurotransmitter binding. Neurotransmitters ...Ligand-gated ion channels mediate signal transduction at chemical synapses and transition between resting, open, and desensitized states in response to neurotransmitter binding. Neurotransmitters that produce maximum open channel probabilities (Po) are full agonists, whereas those that yield lower than maximum Po are partial agonists. Cys-loop receptors are an important class of neurotransmitter receptors, yet a structure-based understanding of the mechanism of partial agonist action has proven elusive. Here, we study the glycine receptor with the full agonist glycine and the partial agonists taurine and γ-amino butyric acid (GABA). We use electrophysiology to show how partial agonists populate agonist-bound, closed channel states and cryo-EM reconstructions to illuminate the structures of intermediate, pre-open states, providing insights into previously unseen conformational states along the receptor reaction pathway. We further correlate agonist-induced conformational changes to Po across members of the receptor family, providing a hypothetical mechanism for partial and full agonist action at Cys-loop receptors. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_20383.map.gz emd_20383.map.gz | 61.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-20383-v30.xml emd-20383-v30.xml emd-20383.xml emd-20383.xml | 9.9 KB 9.9 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_20383_fsc.xml emd_20383_fsc.xml | 11.4 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_20383.png emd_20383.png | 70 KB | ||
| Filedesc metadata |  emd-20383.cif.gz emd-20383.cif.gz | 5.5 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20383 http://ftp.pdbj.org/pub/emdb/structures/EMD-20383 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20383 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20383 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_20383_validation.pdf.gz emd_20383_validation.pdf.gz | 541 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_20383_full_validation.pdf.gz emd_20383_full_validation.pdf.gz | 540.6 KB | 表示 | |
| XML形式データ |  emd_20383_validation.xml.gz emd_20383_validation.xml.gz | 13 KB | 表示 | |
| CIF形式データ |  emd_20383_validation.cif.gz emd_20383_validation.cif.gz | 17.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20383 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20383 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20383 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20383 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  6pm1MC  6ploC  6plpC  6plqC  6plrC  6plsC  6pltC  6pluC  6plvC  6plwC  6plxC  6plyC  6plzC  6pm0C  6pm2C  6pm3C  6pm4C  6pm5C  6pm6C  6pxdC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_20383.map.gz / 形式: CCP4 / 大きさ: 125 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_20383.map.gz / 形式: CCP4 / 大きさ: 125 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | CryoEM structure of zebra fish alpha-1 glycine receptor bound with Taurine in SMA, desensitized state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.823 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : glycine receptor in complex with Taurine
| 全体 | 名称: glycine receptor in complex with Taurine |
|---|---|
| 要素 |
|
-超分子 #1: glycine receptor in complex with Taurine
| 超分子 | 名称: glycine receptor in complex with Taurine / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 250 KDa |
-分子 #1: Glycine receptor subunit alphaZ1
| 分子 | 名称: Glycine receptor subunit alphaZ1 / タイプ: protein_or_peptide / ID: 1 / コピー数: 5 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 52.537598 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN ...文字列: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN FPMDVQTCIM QLESFGYTMN DLIFEWDEKG AVQVADGLTL PQFILKEEKD LRYCTKHYNT GKFTCIEARF HL ERQMGYY LIQMYIPSLL IVILSWVSFW INMDAAPARV GLGITTVLTM TTQSSGSRAS LPKVSYVKAI DIWMAVCLLF VFS ALLEYA AVNFIARQHK ELLRFQRRRR HLKEDEAGDG RFSFAAYGMG PACLQAKDGM AIKGNNNNAP TSTNPPEKTV EEMR KLFIS RAKRIDTVSR VAFPLVFLIF NIFYWITYKI IRSEDIHKQL VPRGSHHHHH HHH UniProtKB: Glycine receptor subunit alphaZ1 |
-分子 #3: 2-AMINOETHANESULFONIC ACID
| 分子 | 名称: 2-AMINOETHANESULFONIC ACID / タイプ: ligand / ID: 3 / コピー数: 5 / 式: TAU |
|---|---|
| 分子量 | 理論値: 125.147 Da |
| Chemical component information | 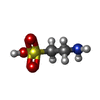 ChemComp-TAU: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 8 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 平均電子線量: 40.8 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















