+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20328 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of alpha-synuclein H50Q Narrow Fibril | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Alpha-synuclein / amyloid / H50Q / hereditary mutation / fibril / PROTEIN FIBRIL | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of phospholipase activity / negative regulation of monooxygenase activity / negative regulation of mitochondrial electron transport, NADH to ubiquinone / positive regulation of glutathione peroxidase activity / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process ...regulation of phospholipase activity / negative regulation of monooxygenase activity / negative regulation of mitochondrial electron transport, NADH to ubiquinone / positive regulation of glutathione peroxidase activity / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / negative regulation of transporter activity / mitochondrial membrane organization / negative regulation of chaperone-mediated autophagy / regulation of reactive oxygen species biosynthetic process / regulation of synaptic vesicle recycling / negative regulation of platelet-derived growth factor receptor signaling pathway / positive regulation of protein localization to cell periphery / negative regulation of exocytosis / regulation of glutamate secretion / response to iron(II) ion / regulation of norepinephrine uptake / SNARE complex assembly / positive regulation of neurotransmitter secretion / dopamine biosynthetic process / regulation of locomotion / positive regulation of inositol phosphate biosynthetic process / synaptic vesicle priming / regulation of macrophage activation / negative regulation of microtubule polymerization / synaptic vesicle transport / dynein complex binding / dopamine uptake involved in synaptic transmission / positive regulation of receptor recycling / regulation of dopamine secretion / protein kinase inhibitor activity / negative regulation of thrombin-activated receptor signaling pathway / cuprous ion binding / response to magnesium ion / response to type II interferon / positive regulation of exocytosis / synaptic vesicle exocytosis / positive regulation of endocytosis / kinesin binding / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / mitochondrial ATP synthesis coupled electron transport / synaptic vesicle endocytosis / regulation of presynapse assembly / negative regulation of serotonin uptake / alpha-tubulin binding / phospholipid metabolic process / supramolecular fiber organization / axon terminus / inclusion body / cellular response to copper ion / cellular response to epinephrine stimulus / Hsp70 protein binding / response to interleukin-1 / : / adult locomotory behavior / positive regulation of release of sequestered calcium ion into cytosol / SNARE binding / excitatory postsynaptic potential / fatty acid metabolic process / long-term synaptic potentiation / phosphoprotein binding / protein tetramerization / regulation of transmembrane transporter activity / protein destabilization / negative regulation of protein kinase activity / microglial cell activation / regulation of long-term neuronal synaptic plasticity / synapse organization / ferrous iron binding / positive regulation of protein serine/threonine kinase activity / tau protein binding / PKR-mediated signaling / receptor internalization / : / phospholipid binding / synaptic vesicle membrane / positive regulation of inflammatory response / actin cytoskeleton / positive regulation of peptidyl-serine phosphorylation / actin binding / cell cortex / cellular response to oxidative stress / histone binding / growth cone / chemical synaptic transmission / neuron apoptotic process / negative regulation of neuron apoptotic process / postsynapse / response to lipopolysaccharide / amyloid fibril formation / molecular adaptor activity / lysosome / transcription cis-regulatory region binding / oxidoreductase activity / positive regulation of apoptotic process Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
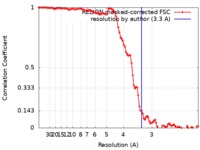
| Method | helical reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||
 Authors Authors | Boyer DR / Li B | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2019 Journal: Nat Struct Mol Biol / Year: 2019Title: Structures of fibrils formed by α-synuclein hereditary disease mutant H50Q reveal new polymorphs. Authors: David R Boyer / Binsen Li / Chuanqi Sun / Weijia Fan / Michael R Sawaya / Lin Jiang / David S Eisenberg /  Abstract: Deposits of amyloid fibrils of α-synuclein are the histological hallmarks of Parkinson's disease, dementia with Lewy bodies and multiple system atrophy, with hereditary mutations in α-synuclein ...Deposits of amyloid fibrils of α-synuclein are the histological hallmarks of Parkinson's disease, dementia with Lewy bodies and multiple system atrophy, with hereditary mutations in α-synuclein linked to the first two of these conditions. Seeing the changes to the structures of amyloid fibrils bearing these mutations may help to understand these diseases. To this end, we determined the cryo-EM structures of α-synuclein fibrils containing the H50Q hereditary mutation. We find that the H50Q mutation results in two previously unobserved polymorphs of α-synuclein: narrow and wide fibrils, formed from either one or two protofilaments, respectively. These structures recapitulate conserved features of the wild-type fold but reveal new structural elements, including a previously unobserved hydrogen-bond network and surprising new protofilament arrangements. The structures of the H50Q polymorphs help to rationalize the faster aggregation kinetics, higher seeding capacity in biosensor cells and greater cytotoxicity that we observe for H50Q compared to wild-type α-synuclein. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20328.map.gz emd_20328.map.gz | 7.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20328-v30.xml emd-20328-v30.xml emd-20328.xml emd-20328.xml | 10.1 KB 10.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20328_fsc.xml emd_20328_fsc.xml | 10.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_20328.png emd_20328.png | 181.1 KB | ||
| Filedesc metadata |  emd-20328.cif.gz emd-20328.cif.gz | 5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20328 http://ftp.pdbj.org/pub/emdb/structures/EMD-20328 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20328 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20328 | HTTPS FTP |
-Validation report
| Summary document |  emd_20328_validation.pdf.gz emd_20328_validation.pdf.gz | 565.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20328_full_validation.pdf.gz emd_20328_full_validation.pdf.gz | 565.4 KB | Display | |
| Data in XML |  emd_20328_validation.xml.gz emd_20328_validation.xml.gz | 10.9 KB | Display | |
| Data in CIF |  emd_20328_validation.cif.gz emd_20328_validation.cif.gz | 14.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20328 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20328 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20328 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20328 | HTTPS FTP |
-Related structure data
| Related structure data |  6peoMC  6pesC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10632 (Title: Cryo electron microscopy of alpha-synuclein H50Q fibrils EMPIAR-10632 (Title: Cryo electron microscopy of alpha-synuclein H50Q fibrilsData size: 595.3 Data #1: Unaligned K2 movies of alpha-synuclein H50Q fibrils [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20328.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20328.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Alpha-synuclein amyloid fibril with H50Q hereditary mutation - Na...
| Entire | Name: Alpha-synuclein amyloid fibril with H50Q hereditary mutation - Narrow Fibril polymorph |
|---|---|
| Components |
|
-Supramolecule #1: Alpha-synuclein amyloid fibril with H50Q hereditary mutation - Na...
| Supramolecule | Name: Alpha-synuclein amyloid fibril with H50Q hereditary mutation - Narrow Fibril polymorph type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Alpha-synuclein
| Macromolecule | Name: Alpha-synuclein / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.466091 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDVFMKGLSK AKEGVVAAAE KTKQGVAEAA GKTKEGVLYV GSKTKEGVVQ GVATVAEKTK EQVTNVGGAV VTGVTAVAQK TVEGAGSIA AATGFVKKDQ LGKNEEGAPQ EGILEDMPVD PDNEAYEMPS EEGYQDYEPE A UniProtKB: Alpha-synuclein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 26.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)