+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | New1 bound to 80S ribosome; eRF1, P-tRNA, E-tRNA (State 7) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RIBOSOME / New1 / Translation termination / 80S | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.3 Å | |||||||||
 Authors Authors | Paternoga H / Pochopien AA / Wilson DN | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation | Journal: bioRxiv / Year: 2024 Title: The ABCF ATPase New1 resolves translation termination defects associated with specific tRNA and tRNA isoacceptors in the P site. Authors: Kathryn Turnbull / Helge Paternoga / Esther von der Weth / Artyom A Egorov / Agnieszka A Pochopien / Yujie Zhang / Lilit Nersisyan / Tõnu Margus / Marcus J O Johansson / Vicent Pelechano / ...Authors: Kathryn Turnbull / Helge Paternoga / Esther von der Weth / Artyom A Egorov / Agnieszka A Pochopien / Yujie Zhang / Lilit Nersisyan / Tõnu Margus / Marcus J O Johansson / Vicent Pelechano / Daniel N Wilson / Vasili Hauryliuk /     Abstract: The efficiency of translation termination is determined by the nature of the stop codon as well as its context. In eukaryotes, recognition of the A-site stop codon and release of the polypeptide are ...The efficiency of translation termination is determined by the nature of the stop codon as well as its context. In eukaryotes, recognition of the A-site stop codon and release of the polypeptide are mediated by release factors eRF1 and eRF3, respectively. Translation termination is modulated by other factors which either directly interact with release factors or bind to the E-site and modulate the activity of the peptidyl transferase center. Previous studies suggested that the ABCF ATPase New1 is involved in translation termination and/or ribosome recycling, however, the exact function remained unclear. Here, we have applied 5PSeq, single-particle cryo-EM and readthrough reporter assays to provide insight into the biological function of New1. We show that the lack of New1 results in ribosomal stalling at stop codons preceded by a lysine or arginine codon and that the stalling is not defined by the nature of the C-terminal amino acid but rather by the identity of the tRNA isoacceptor in the P-site. Collectively, our results suggest that translation termination is inefficient when ribosomes have specific tRNA isoacceptors in the P-site and that the recruitment of New1 rescues ribosomes at these problematic termination contexts. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19924.map.gz emd_19924.map.gz | 224.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19924-v30.xml emd-19924-v30.xml emd-19924.xml emd-19924.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_19924.png emd_19924.png | 76.4 KB | ||
| Filedesc metadata |  emd-19924.cif.gz emd-19924.cif.gz | 4.5 KB | ||
| Others |  emd_19924_additional_1.map.gz emd_19924_additional_1.map.gz emd_19924_half_map_1.map.gz emd_19924_half_map_1.map.gz emd_19924_half_map_2.map.gz emd_19924_half_map_2.map.gz | 83.3 MB 226.4 MB 225.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19924 http://ftp.pdbj.org/pub/emdb/structures/EMD-19924 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19924 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19924 | HTTPS FTP |
-Validation report
| Summary document |  emd_19924_validation.pdf.gz emd_19924_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_19924_full_validation.pdf.gz emd_19924_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_19924_validation.xml.gz emd_19924_validation.xml.gz | 16.6 KB | Display | |
| Data in CIF |  emd_19924_validation.cif.gz emd_19924_validation.cif.gz | 19.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19924 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19924 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19924 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19924 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_19924.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19924.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||
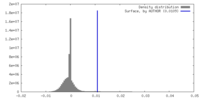
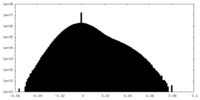
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Local resolution filtered map
| File | emd_19924_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution filtered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
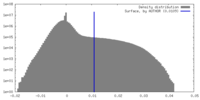
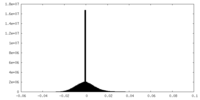
| Density Histograms |
-Half map: #1
| File | emd_19924_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
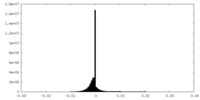
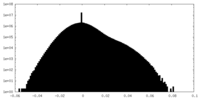
| Density Histograms |
-Half map: #2
| File | emd_19924_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
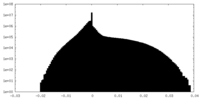
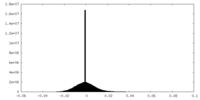
| Density Histograms |
- Sample components
Sample components
-Entire : Ex vivo pullout of New1
| Entire | Name: Ex vivo pullout of New1 |
|---|---|
| Components |
|
-Supramolecule #1: Ex vivo pullout of New1
| Supramolecule | Name: Ex vivo pullout of New1 / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R3/3 / Material: COPPER / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 2 |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 5.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 5613 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)