+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mouse RPL39 integrated into the yeast 60S ribosomal subunit | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | 60S ribosomal subunit / protein exit tunnel / RPL39 / RPL39L / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationFormation of a pool of free 40S subunits / SRP-dependent cotranslational protein targeting to membrane / Major pathway of rRNA processing in the nucleolus and cytosol / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / L13a-mediated translational silencing of Ceruloplasmin expression / GTP hydrolysis and joining of the 60S ribosomal subunit / hexon binding / pre-mRNA 5'-splice site binding / cleavage in ITS2 between 5.8S rRNA and LSU-rRNA of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) ...Formation of a pool of free 40S subunits / SRP-dependent cotranslational protein targeting to membrane / Major pathway of rRNA processing in the nucleolus and cytosol / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / L13a-mediated translational silencing of Ceruloplasmin expression / GTP hydrolysis and joining of the 60S ribosomal subunit / hexon binding / pre-mRNA 5'-splice site binding / cleavage in ITS2 between 5.8S rRNA and LSU-rRNA of tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / response to cycloheximide / SRP-dependent cotranslational protein targeting to membrane / GTP hydrolysis and joining of the 60S ribosomal subunit / Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / Formation of a pool of free 40S subunits / negative regulation of mRNA splicing, via spliceosome / preribosome, large subunit precursor / L13a-mediated translational silencing of Ceruloplasmin expression / translational elongation / ribosomal large subunit export from nucleus / regulation of translational fidelity / protein-RNA complex assembly / translational termination / maturation of LSU-rRNA / maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / ribosomal large subunit biogenesis / translational initiation / macroautophagy / maintenance of translational fidelity / modification-dependent protein catabolic process / protein tag activity / rRNA processing / ribosome biogenesis / viral capsid / 5S rRNA binding / large ribosomal subunit rRNA binding / ribosomal large subunit assembly / cytoplasmic translation / cytosolic large ribosomal subunit / negative regulation of translation / rRNA binding / ribosome / protein ubiquitination / structural constituent of ribosome / translation / response to antibiotic / mRNA binding / ubiquitin protein ligase binding / host cell nucleus / nucleolus / RNA binding / nucleus / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
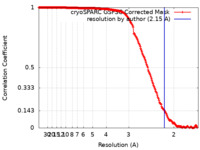
| Method | single particle reconstruction / cryo EM / Resolution: 2.15 Å | |||||||||
 Authors Authors | Rabl J / Banerjee A / Boehringer D / Zavolan M | |||||||||
| Funding support |  Switzerland, 1 items Switzerland, 1 items
| |||||||||
 Citation Citation | Journal: Acta Crystallogr D Struct Biol / Year: 2018 Title: Real-space refinement in PHENIX for cryo-EM and crystallography. Authors: Pavel V Afonine / Billy K Poon / Randy J Read / Oleg V Sobolev / Thomas C Terwilliger / Alexandre Urzhumtsev / Paul D Adams /    Abstract: This article describes the implementation of real-space refinement in the phenix.real_space_refine program from the PHENIX suite. The use of a simplified refinement target function enables very fast ...This article describes the implementation of real-space refinement in the phenix.real_space_refine program from the PHENIX suite. The use of a simplified refinement target function enables very fast calculation, which in turn makes it possible to identify optimal data-restraint weights as part of routine refinements with little runtime cost. Refinement of atomic models against low-resolution data benefits from the inclusion of as much additional information as is available. In addition to standard restraints on covalent geometry, phenix.real_space_refine makes use of extra information such as secondary-structure and rotamer-specific restraints, as well as restraints or constraints on internal molecular symmetry. The re-refinement of 385 cryo-EM-derived models available in the Protein Data Bank at resolutions of 6 Å or better shows significant improvement of the models and of the fit of these models to the target maps. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17550.map.gz emd_17550.map.gz | 775.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17550-v30.xml emd-17550-v30.xml emd-17550.xml emd-17550.xml | 61.6 KB 61.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17550_fsc.xml emd_17550_fsc.xml | 19.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_17550.png emd_17550.png | 149.6 KB | ||
| Masks |  emd_17550_msk_1.map emd_17550_msk_1.map | 824 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17550.cif.gz emd-17550.cif.gz | 13.5 KB | ||
| Others |  emd_17550_half_map_1.map.gz emd_17550_half_map_1.map.gz emd_17550_half_map_2.map.gz emd_17550_half_map_2.map.gz | 764 MB 763.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17550 http://ftp.pdbj.org/pub/emdb/structures/EMD-17550 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17550 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17550 | HTTPS FTP |
-Validation report
| Summary document |  emd_17550_validation.pdf.gz emd_17550_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_17550_full_validation.pdf.gz emd_17550_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_17550_validation.xml.gz emd_17550_validation.xml.gz | 28.3 KB | Display | |
| Data in CIF |  emd_17550_validation.cif.gz emd_17550_validation.cif.gz | 37.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17550 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17550 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17550 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17550 | HTTPS FTP |
-Related structure data
| Related structure data |  8p8nMC  8p8mC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17550.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17550.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17550_msk_1.map emd_17550_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_17550_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17550_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Mouse RPL39 integrated into yeast 60S ribosomal subunit
+Supramolecule #1: Mouse RPL39 integrated into yeast 60S ribosomal subunit
+Macromolecule #1: Large ribosomal subunit protein eL39
+Macromolecule #2: 60S ribosomal protein L7-A
+Macromolecule #3: 60S ribosomal protein L25
+Macromolecule #4: 60S ribosomal protein L38
+Macromolecule #6: 60S ribosomal protein L8-A
+Macromolecule #7: 60S ribosomal protein L26-A
+Macromolecule #8: Ubiquitin-60S ribosomal protein L40
+Macromolecule #9: 60S ribosomal protein L13-A
+Macromolecule #10: 60S ribosomal protein L9-A
+Macromolecule #11: 60S ribosomal protein L27-A
+Macromolecule #12: 60S ribosomal protein L42-A
+Macromolecule #13: 60S ribosomal protein L14-A
+Macromolecule #14: 60S ribosomal protein L10
+Macromolecule #15: 60S ribosomal protein L28
+Macromolecule #16: 60S ribosomal protein L43-A
+Macromolecule #17: 60S ribosomal protein L15-A
+Macromolecule #18: 60S ribosomal protein L11-A
+Macromolecule #19: 60S ribosomal protein L29
+Macromolecule #20: 60S ribosomal protein L17-A
+Macromolecule #21: 60S ribosomal protein L16-A
+Macromolecule #22: 60S ribosomal protein L18-A
+Macromolecule #23: 60S ribosomal protein L30
+Macromolecule #24: 60S ribosomal protein L19-A
+Macromolecule #26: 60S ribosomal protein L20-A
+Macromolecule #27: 60S ribosomal protein L31-A
+Macromolecule #28: 60S ribosomal protein L21-A
+Macromolecule #30: 60S ribosomal protein L22-A
+Macromolecule #31: 60S ribosomal protein L32
+Macromolecule #32: 60S ribosomal protein L23-A
+Macromolecule #33: 60S ribosomal protein L2-A
+Macromolecule #34: 60S ribosomal protein L24-A
+Macromolecule #35: 60S ribosomal protein L33-A
+Macromolecule #36: 60S ribosomal protein L3
+Macromolecule #37: 60S ribosomal protein L34-A
+Macromolecule #38: 60S ribosomal protein L4-A
+Macromolecule #39: 60S ribosomal protein L35-A
+Macromolecule #40: 60S ribosomal protein L5
+Macromolecule #41: 60S ribosomal protein L36-A
+Macromolecule #42: 60S ribosomal protein L6-A
+Macromolecule #43: 60S ribosomal protein L37-A
+Macromolecule #5: 25S rRNA
+Macromolecule #25: 5.8S rRNA
+Macromolecule #29: 5S rRNA
+Macromolecule #44: MAGNESIUM ION
+Macromolecule #45: CHLORIDE ION
+Macromolecule #46: SPERMIDINE
+Macromolecule #47: SPERMINE
+Macromolecule #48: ZINC ION
+Macromolecule #49: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER |
|---|---|
| Output model |  PDB-8p8n: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)