[English] 日本語
 Yorodumi
Yorodumi- EMDB-17387: Full-length bacterial polysaccharide co-polymerase WzzE mutant R2... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Full-length bacterial polysaccharide co-polymerase WzzE mutant R267A from E. coli. C4 symmetry | ||||||||||||
 Map data Map data | E. coli WzzE mutant R267A, C4 symmetry, unsharpened | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Complex / Lipopolysaccharide / bacterial polysaccharide co-polymerase / MEMBRANE PROTEIN | ||||||||||||
| Function / homology | ECA polysaccharide chain length modulation protein WzzE / enterobacterial common antigen biosynthetic process / Polysaccharide chain length determinant N-terminal domain / Chain length determinant protein / : / protein tyrosine kinase activity / plasma membrane / ECA polysaccharide chain length modulation protein Function and homology information Function and homology information | ||||||||||||
| Biological species |  | ||||||||||||
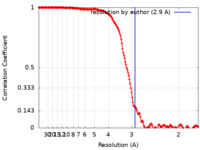
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | ||||||||||||
 Authors Authors | Wiseman B / Hogbom M | ||||||||||||
| Funding support |  Sweden, 3 items Sweden, 3 items
| ||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2023 Journal: Commun Biol / Year: 2023Title: Alternating L4 loop architecture of the bacterial polysaccharide co-polymerase WzzE. Authors: Benjamin Wiseman / Göran Widmalm / Martin Högbom /  Abstract: Lipopolysaccharides such as the enterobacterial common antigen are important components of the enterobacterial cell envelope that act as a protective barrier against the environment and are often ...Lipopolysaccharides such as the enterobacterial common antigen are important components of the enterobacterial cell envelope that act as a protective barrier against the environment and are often polymerized by the inner membrane bound Wzy-dependent pathway. By employing cryo-electron microscopy we show that WzzE, the co-polymerase component of this pathway that is responsible for the length modulation of the enterobacterial common antigen, is octameric with alternating up-down conformations of its L4 loops. The alternating up-down nature of these essential loops, located at the top of the periplasmic bell, are modulated by clashing helical faces between adjacent protomers that flank the L4 loops around the octameric periplasmic bell. This alternating arrangement and a highly negatively charged binding face create a dynamic environment in which the polysaccharide chain is extended, and suggest a ratchet-type mechanism for polysaccharide elongation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17387.map.gz emd_17387.map.gz | 194.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17387-v30.xml emd-17387-v30.xml emd-17387.xml emd-17387.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17387_fsc.xml emd_17387_fsc.xml | 17.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_17387.png emd_17387.png | 83.6 KB | ||
| Filedesc metadata |  emd-17387.cif.gz emd-17387.cif.gz | 6 KB | ||
| Others |  emd_17387_additional_1.map.gz emd_17387_additional_1.map.gz emd_17387_half_map_1.map.gz emd_17387_half_map_1.map.gz emd_17387_half_map_2.map.gz emd_17387_half_map_2.map.gz | 373.6 MB 367.2 MB 367.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17387 http://ftp.pdbj.org/pub/emdb/structures/EMD-17387 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17387 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17387 | HTTPS FTP |
-Validation report
| Summary document |  emd_17387_validation.pdf.gz emd_17387_validation.pdf.gz | 731.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_17387_full_validation.pdf.gz emd_17387_full_validation.pdf.gz | 731 KB | Display | |
| Data in XML |  emd_17387_validation.xml.gz emd_17387_validation.xml.gz | 25 KB | Display | |
| Data in CIF |  emd_17387_validation.cif.gz emd_17387_validation.cif.gz | 32.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17387 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17387 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17387 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17387 | HTTPS FTP |
-Related structure data
| Related structure data |  8p3oMC  8bhwC  8p3pC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_17387.map.gz / Format: CCP4 / Size: 396.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17387.map.gz / Format: CCP4 / Size: 396.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli WzzE mutant R267A, C4 symmetry, unsharpened | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8676 Å | ||||||||||||||||||||||||||||||||||||
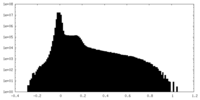
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: E. coli WzzE mutant R267A, C4 symmetry, sharpened
| File | emd_17387_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli WzzE mutant R267A, C4 symmetry, sharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
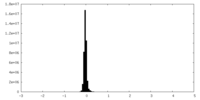
| Density Histograms |
-Half map: E. coli WzzE mutant R267A, C4 symmetry, half map B
| File | emd_17387_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli WzzE mutant R267A, C4 symmetry, half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
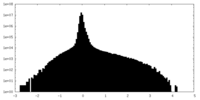
| Density Histograms |
-Half map: E. coli WzzE mutant R267A, C4 symmetry, half map A
| File | emd_17387_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | E. coli WzzE mutant R267A, C4 symmetry, half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
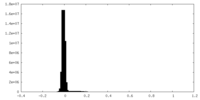
| Density Histograms |
- Sample components
Sample components
-Entire : Bacterial Polysaccharide Co-polymerase WzzE
| Entire | Name: Bacterial Polysaccharide Co-polymerase WzzE |
|---|---|
| Components |
|
-Supramolecule #1: Bacterial Polysaccharide Co-polymerase WzzE
| Supramolecule | Name: Bacterial Polysaccharide Co-polymerase WzzE / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Homo octameric complex |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 320 KDa |
-Macromolecule #1: ECA polysaccharide chain length modulation protein
| Macromolecule | Name: ECA polysaccharide chain length modulation protein / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.324906 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTQPMPGKPA EDAENELDIR GLFRTLWAGK LWIIGMGLAF ALIALAYTFF ARQEWSSTAI TDRPTVNMLG GYYSQQQFLR NLDVRSNMA SADQPSVMDE AYKEFVMQLA SWDTRREFWL QTDYYKQRMV GNSKADAALL DEMINNIQFI PGDFTRAVND S VKLIAETA ...String: MTQPMPGKPA EDAENELDIR GLFRTLWAGK LWIIGMGLAF ALIALAYTFF ARQEWSSTAI TDRPTVNMLG GYYSQQQFLR NLDVRSNMA SADQPSVMDE AYKEFVMQLA SWDTRREFWL QTDYYKQRMV GNSKADAALL DEMINNIQFI PGDFTRAVND S VKLIAETA PDANNLLRQY VAFASQRAAS HLNDELKGAW AARTIQMKAQ VKRQEEVAKA IYDRRMNSIE QALKIAEQHN IS RSATDVP AEELPDSEMF LLGRPMLQAA LENLQAVGPA FDLDYDQNRA MLNTLNVGPT LDPRFQTYRY LRTPEEPVKR DSP RRAFLM IMWGIVGGLI GAGVALTRRC SKEFRVPGSH HHHHHHH UniProtKB: ECA polysaccharide chain length modulation protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.0 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: C-flat-2/2 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 9573 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)