[English] 日本語
 Yorodumi
Yorodumi- EMDB-16296: Cryo-EM structure of native nanodisc-reconstituted wildtype human... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of native nanodisc-reconstituted wildtype human MRP4 (inward-facing conformation) - no nucleotides/substrates added | |||||||||
 Map data Map data | Cryo-EM map of wild type human MRP4 in native nanodiscs | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / ABCC4 / MRP4 / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationguanine nucleotide transmembrane transporter activity / 15-hydroxyprostaglandin dehydrogenase (NAD+) activity / purine nucleotide transmembrane transporter activity / cAMP transport / ABC-type bile acid transporter activity / platelet dense granule membrane / leukotriene transport / urate transport / platelet degranulation / prostaglandin transport ...guanine nucleotide transmembrane transporter activity / 15-hydroxyprostaglandin dehydrogenase (NAD+) activity / purine nucleotide transmembrane transporter activity / cAMP transport / ABC-type bile acid transporter activity / platelet dense granule membrane / leukotriene transport / urate transport / platelet degranulation / prostaglandin transport / glutathione transmembrane transporter activity / ABC-type glutathione-S-conjugate transporter / ABC-type glutathione S-conjugate transporter activity / prostaglandin transmembrane transporter activity / ATPase-coupled inorganic anion transmembrane transporter activity / urate transmembrane transporter activity / external side of apical plasma membrane / xenobiotic transmembrane transport / export across plasma membrane / ABC-type xenobiotic transporter / prostaglandin secretion / Paracetamol ADME / Translocases; Catalysing the translocation of other compounds; Linked to the hydrolysis of a nucleoside triphosphate / ABC-type xenobiotic transporter activity / Azathioprine ADME / bile acid and bile salt transport / efflux transmembrane transporter activity / xenobiotic transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / transport across blood-brain barrier / cilium assembly / ABC-type transporter activity / xenobiotic metabolic process / ABC-family proteins mediated transport / transmembrane transport / Platelet degranulation / basolateral plasma membrane / apical plasma membrane / nucleolus / Golgi apparatus / ATP hydrolysis activity / ATP binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
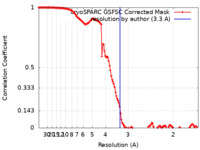
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Bloch MB / Pape TH / Raj I / Taylor NMI | |||||||||
| Funding support |  Denmark, 1 items Denmark, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2023 Journal: Structure / Year: 2023Title: Structural and mechanistic basis of substrate transport by the multidrug transporter MRP4. Authors: Magnus Bloch / Isha Raj / Tillmann Pape / Nicholas M I Taylor /  Abstract: Multidrug resistance-associated protein 4 (MRP4) is an ATP-binding cassette (ABC) transporter expressed at multiple tissue barriers where it actively extrudes a wide variety of drug compounds. ...Multidrug resistance-associated protein 4 (MRP4) is an ATP-binding cassette (ABC) transporter expressed at multiple tissue barriers where it actively extrudes a wide variety of drug compounds. Overexpression of MRP4 provides resistance to clinically used antineoplastic agents, making it a highly attractive therapeutic target for countering multidrug resistance. Here, we report cryo-EM structures of multiple physiologically relevant states of lipid bilayer-embedded human MRP4, including complexes between MRP4 and two widely used chemotherapeutic agents and a complex between MRP4 and its native substrate. The structures display clear similarities and distinct differences in the coordination of these chemically diverse substrates and, in combination with functional and mutational analysis, reveal molecular details of the transport mechanism. Our study provides key insights into the unusually broad substrate specificity of MRP4 and constitutes an important contribution toward a general understanding of multidrug transporters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16296.map.gz emd_16296.map.gz | 203.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16296-v30.xml emd-16296-v30.xml emd-16296.xml emd-16296.xml | 16.3 KB 16.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16296_fsc.xml emd_16296_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_16296.png emd_16296.png | 85.4 KB | ||
| Masks |  emd_16296_msk_1.map emd_16296_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16296.cif.gz emd-16296.cif.gz | 5.6 KB | ||
| Others |  emd_16296_half_map_1.map.gz emd_16296_half_map_1.map.gz emd_16296_half_map_2.map.gz emd_16296_half_map_2.map.gz | 200.3 MB 200.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16296 http://ftp.pdbj.org/pub/emdb/structures/EMD-16296 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16296 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16296 | HTTPS FTP |
-Validation report
| Summary document |  emd_16296_validation.pdf.gz emd_16296_validation.pdf.gz | 798.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16296_full_validation.pdf.gz emd_16296_full_validation.pdf.gz | 798.3 KB | Display | |
| Data in XML |  emd_16296_validation.xml.gz emd_16296_validation.xml.gz | 21.6 KB | Display | |
| Data in CIF |  emd_16296_validation.cif.gz emd_16296_validation.cif.gz | 27.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16296 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16296 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16296 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16296 | HTTPS FTP |
-Related structure data
| Related structure data |  8bjfC  8bwoC  8bwpC  8bwqC  8bwrC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16296.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16296.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of wild type human MRP4 in native nanodiscs | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.832 Å | ||||||||||||||||||||||||||||||||||||
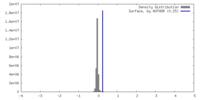
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16296_msk_1.map emd_16296_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
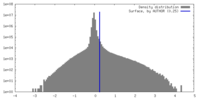
| Density Histograms |
-Half map: Half map 1 of wild type human MRP4 in native nanodiscs
| File | emd_16296_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 of wild type human MRP4 in native nanodiscs | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 of wild type human MRP4 in native nanodiscs
| File | emd_16296_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 of wild type human MRP4 in native nanodiscs | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of native nanodisc-reconstituted wildtype human...
| Entire | Name: Cryo-EM structure of native nanodisc-reconstituted wildtype human MRP4 (inward-facing conformation) - no nucleotides/substrates added |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of native nanodisc-reconstituted wildtype human...
| Supramolecule | Name: Cryo-EM structure of native nanodisc-reconstituted wildtype human MRP4 (inward-facing conformation) - no nucleotides/substrates added type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Human ATP-binding cassette sub-family C member 4
| Macromolecule | Name: Human ATP-binding cassette sub-family C member 4 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: ABC-type xenobiotic transporter |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLPVYQEVKP NPLQDANLCS RVFFWWLNPL FKIGHKRRLE EDDMYSVLPE DRSQHLGEEL QGFWDKEVL RAENDAQKPS LTRAIIKCYW KSYLVLGIFT LIEESAKVIQ PIFLGKIINY F ENYDPMDS VALNTAYAYA TVLTFCTLIL AILHHLYFYH VQCAGMRLRV ...String: MLPVYQEVKP NPLQDANLCS RVFFWWLNPL FKIGHKRRLE EDDMYSVLPE DRSQHLGEEL QGFWDKEVL RAENDAQKPS LTRAIIKCYW KSYLVLGIFT LIEESAKVIQ PIFLGKIINY F ENYDPMDS VALNTAYAYA TVLTFCTLIL AILHHLYFYH VQCAGMRLRV AMCHMIYRKA LR LSNMAMG KTTTGQIVNL LSNDVNKFDQ VTVFLHFLWA GPLQAIAVTA LLWMEIGISC LAG MAVLII LLPLQSCFGK LFSSLRSKTA TFTDARIRTM NEVITGIRII KMYAWEKSFS NLIT NLRKK EISKILRSSC LRGMNLASFF SASKIIVFVT FTTYVLLGSV ITASRVFVAV TLYGA VRLT VTLFFPSAIE RVSEAIVSIR RIQTFLLLDE ISQRNRQLPS DGKKMVHVQD FTAFWD KAS ETPTLQGLSF TVRPGELLAV VGPVGAGKSS LLSAVLGELA PSHGLVSVHG RIAYVSQ QP WVFSGTLRSN ILFGKKYEKE RYEKVIKACA LKKDLQLLED GDLTVIGDRG TTLSGGQK A RVNLARAVYQ DADIYLLDDP LSAVDAEVSR HLFELCICQI LHEKITILVT HQLQYLKAA SQILILKDGK MVQKGTYTEF LKSGIDFGSL LKKDNEESEQ PPVPGTPTLR NRTFSESSVW SQQSSRPSL KDGALESQDT ENVPVTLSEE NRSEGKVGFQ AYKNYFRAGA HWIVFIFLIL L NTAAQVAY VLQDWWLSYW ANKQSMLNVT VNGGGNVTEK LDLNWYLGIY SGLTVATVLF GI ARSLLVF YVLVNSSQTL HNKMFESILK APVLFFDRNP IGRILNRFSK DIGHLDDLLP LTF LDFIQT LLQVVGVVSV AVAVIPWIAI PLVPLGIIFI FLRRYFLETS RDVKRLESTT RSPV FSHLS SSLQGLWTIR AYKAEERCQE LFDAHQDLHS EAWFLFLTTS RWFAVRLDAI CAMFV IIVA FGSLILAKTL DAGQVGLALS YALTLMGMFQ WCVRQSAEVE NMMISVERVI EYTDLE KEA PWEYQKRPPP AWPHEGVIIF DNVNFMYSPG GPLVLKHLTA LIKSQEKVGI VGRTGAG KS SLISALFRLS EPEGKIWIDK ILTTEIGLHD LRKKMSIIPQ EPVLFTGTMR KNLDPFNE H TDEELWNALQ EVQLKETIED LPGKMDTELA ESGSNFSVGQ RQLVCLARAI LRKNQILII DEATANVDPR TDELIQKKIR EKFAHCTVLT IAHRLNTIID SDKIMVLDSG RLKEYDEPYV LLQNKESLF YKMVQQLGKA EAAALTETAK QVYFKRNYPH IGHTDHMVTN TSNGQPSTLT I FETAL UniProtKB: ATP-binding cassette sub-family C member 4 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | This sample was monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number real images: 7335 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)