[English] 日本語
 Yorodumi
Yorodumi- EMDB-15721: Mouse heavy chain apoferritin after laser melting and revitrification -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mouse heavy chain apoferritin after laser melting and revitrification | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Globular / Storage / ferritin / METAL BINDING PROTEIN | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 1.59 Å | |||||||||
 Authors Authors | Bongiovanni G / Harder OF / Voss JM / Drabbels M / Lorenz UJ | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2023 Journal: Acta Crystallogr D Struct Biol / Year: 2023Title: Near-atomic resolution reconstructions from in situ revitrified cryo samples. Authors: Gabriele Bongiovanni / Oliver F Harder / Jonathan M Voss / Marcel Drabbels / Ulrich J Lorenz /  Abstract: A microsecond time-resolved version of cryo-electron microscopy (cryo-EM) has recently been introduced to enable observation of the fast conformational motions of proteins. The technique involves ...A microsecond time-resolved version of cryo-electron microscopy (cryo-EM) has recently been introduced to enable observation of the fast conformational motions of proteins. The technique involves locally melting a cryo sample with a laser beam to allow the proteins to undergo dynamics in the liquid phase. When the laser is switched off, the sample cools within just a few microseconds and revitrifies, trapping particles in their transient configurations, in which they can subsequently be imaged. Two alternative implementations of the technique have previously been described, using either an optical microscope or performing revitrification experiments in situ. Here, it is shown that it is possible to obtain near-atomic resolution reconstructions from in situ revitrified cryo samples. Moreover, the resulting map is indistinguishable from that obtained from a conventional sample within the spatial resolution. Interestingly, it is observed that revitrification leads to a more homogeneous angular distribution of the particles, suggesting that revitrification may potentially be used to overcome issues of preferred particle orientation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15721.map.gz emd_15721.map.gz | 633.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15721-v30.xml emd-15721-v30.xml emd-15721.xml emd-15721.xml | 11.2 KB 11.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_15721.png emd_15721.png | 104.4 KB | ||
| Others |  emd_15721_half_map_1.map.gz emd_15721_half_map_1.map.gz emd_15721_half_map_2.map.gz emd_15721_half_map_2.map.gz | 621.7 MB 621.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15721 http://ftp.pdbj.org/pub/emdb/structures/EMD-15721 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15721 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15721 | HTTPS FTP |
-Validation report
| Summary document |  emd_15721_validation.pdf.gz emd_15721_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15721_full_validation.pdf.gz emd_15721_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_15721_validation.xml.gz emd_15721_validation.xml.gz | 19.8 KB | Display | |
| Data in CIF |  emd_15721_validation.cif.gz emd_15721_validation.cif.gz | 23.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15721 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15721 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15721 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15721 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15721.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15721.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.455 Å | ||||||||||||||||||||||||||||||||||||
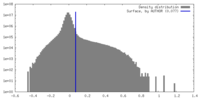
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_15721_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
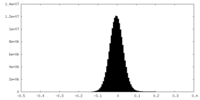
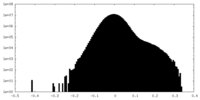
| Density Histograms |
-Half map: #2
| File | emd_15721_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
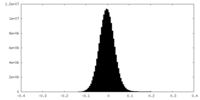
| Density Histograms |
- Sample components
Sample components
-Entire : Mouse heavy-chain apoferritin
| Entire | Name: Mouse heavy-chain apoferritin |
|---|---|
| Components |
|
-Supramolecule #1: Mouse heavy-chain apoferritin
| Supramolecule | Name: Mouse heavy-chain apoferritin / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 1.59 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 72811 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)