[English] 日本語
 Yorodumi
Yorodumi- EMDB-15215: Asymmetric reconstruction of averaged ribosomes from Saccharomyce... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae | ||||||||||||||||||
 Map data Map data | Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | ribosome / replication / active translation / ribosomal states / polysomes | ||||||||||||||||||
| Biological species |  | ||||||||||||||||||
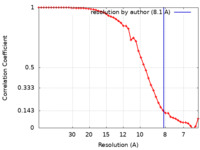
| Method | single particle reconstruction / cryo EM / Resolution: 8.1 Å | ||||||||||||||||||
 Authors Authors | Schmidt L / Tueting C / Kyrilis F / Hamdi F / Semchonok DA / Kastritis PL | ||||||||||||||||||
| Funding support |  Germany, European Union, 5 items Germany, European Union, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Delineating organizational principles of the endogenous L-A virus by cryo-EM and computational analysis of native cell extracts. Authors: Lisa Schmidt / Christian Tüting / Fotis L Kyrilis / Farzad Hamdi / Dmitry A Semchonok / Gerd Hause / Annette Meister / Christian Ihling / Milton T Stubbs / Andrea Sinz / Panagiotis L Kastritis /   Abstract: The high abundance of most viruses in infected host cells benefits their structural characterization. However, endogenous viruses are present in low copy numbers and are therefore challenging to ...The high abundance of most viruses in infected host cells benefits their structural characterization. However, endogenous viruses are present in low copy numbers and are therefore challenging to investigate. Here, we retrieve cell extracts enriched with an endogenous virus, the yeast L-A virus. The determined cryo-EM structure discloses capsid-stabilizing cation-π stacking, widespread across viruses and within the Totiviridae, and an interplay of non-covalent interactions from ten distinct capsomere interfaces. The capsid-embedded mRNA decapping active site trench is supported by a constricting movement of two flexible opposite-facing loops. tRNA-loaded polysomes and other biomacromolecules, presumably mRNA, are found in virus proximity within the cell extract. Mature viruses participate in larger viral communities resembling their rare in-cell equivalents in terms of size, composition, and inter-virus distances. Our results collectively describe a 3D-architecture of a viral milieu, opening the door to cell-extract-based high-resolution structural virology. #1:  Journal: Biorxiv / Year: 2022 Journal: Biorxiv / Year: 2022Title: Delineating organizational principles of the endogenous L-A virus by cryo-EM and computational analysis of native cell extracts Authors: Schmidt L / Tuting C / Kyrilis FL / Hamdi F / Semchonok DA / Hause G / Meister A / Ihling C / Shah PNM / Stubbs MT / Sinz A / Stuart DI / Kastritis PL | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15215.map.gz emd_15215.map.gz | 7.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15215-v30.xml emd-15215-v30.xml emd-15215.xml emd-15215.xml | 31.5 KB 31.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15215_fsc.xml emd_15215_fsc.xml | 5.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_15215.png emd_15215.png | 182.7 KB | ||
| Filedesc metadata |  emd-15215.cif.gz emd-15215.cif.gz | 5.1 KB | ||
| Others |  emd_15215_additional_1.map.gz emd_15215_additional_1.map.gz emd_15215_additional_2.map.gz emd_15215_additional_2.map.gz emd_15215_additional_3.map.gz emd_15215_additional_3.map.gz emd_15215_additional_4.map.gz emd_15215_additional_4.map.gz emd_15215_additional_5.map.gz emd_15215_additional_5.map.gz emd_15215_additional_6.map.gz emd_15215_additional_6.map.gz emd_15215_half_map_1.map.gz emd_15215_half_map_1.map.gz emd_15215_half_map_2.map.gz emd_15215_half_map_2.map.gz | 7.5 MB 7.5 MB 7.5 MB 7.5 MB 7.5 MB 7.5 MB 7.3 MB 7.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15215 http://ftp.pdbj.org/pub/emdb/structures/EMD-15215 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15215 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15215 | HTTPS FTP |
-Validation report
| Summary document |  emd_15215_validation.pdf.gz emd_15215_validation.pdf.gz | 701.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15215_full_validation.pdf.gz emd_15215_full_validation.pdf.gz | 700.7 KB | Display | |
| Data in XML |  emd_15215_validation.xml.gz emd_15215_validation.xml.gz | 10.5 KB | Display | |
| Data in CIF |  emd_15215_validation.cif.gz emd_15215_validation.cif.gz | 14 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15215 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15215 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15215 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15215 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15215.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15215.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.177 Å | ||||||||||||||||||||||||||||||||||||
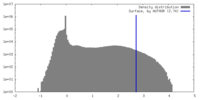
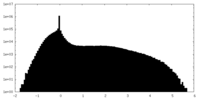
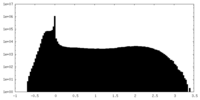
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Reconstructed map of a ribosome after variability analysis...
| File | emd_15215_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstructed map of a ribosome after variability analysis showing tRNA occupation at sites A/A P/P | ||||||||||||
| Projections & Slices |
| ||||||||||||
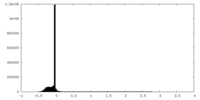
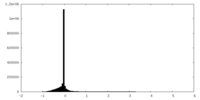
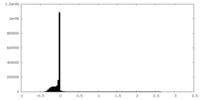
| Density Histograms |
-Additional map: Reconstructed map of a ribosome after variability analysis...
| File | emd_15215_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstructed map of a ribosome after variability analysis showing tRNA occupation at sites P/P E/E | ||||||||||||
| Projections & Slices |
| ||||||||||||
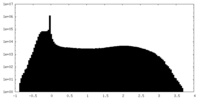
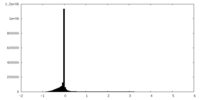
| Density Histograms |
-Additional map: Reconstructed map of a ribosome after variability analysis...
| File | emd_15215_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstructed map of a ribosome after variability analysis showing tRNA occupation at sites P/E E/- | ||||||||||||
| Projections & Slices |
| ||||||||||||
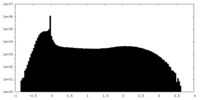
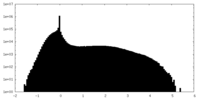
| Density Histograms |
-Additional map: Reconstructed map of a ribosome after variability analysis...
| File | emd_15215_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstructed map of a ribosome after variability analysis showing tRNA occupation at sites A/P P/E | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Reconstructed map of a ribosome after variability analysis...
| File | emd_15215_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstructed map of a ribosome after variability analysis showing tRNA occupation at site A/P | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Reconstructed map of a ribosome after variability analysis...
| File | emd_15215_additional_6.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstructed map of a ribosome after variability analysis showing tRNA occupation at site A/A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae...
| File | emd_15215_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae - Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
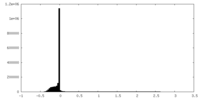
| Density Histograms |
-Half map: Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae...
| File | emd_15215_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asymmetric reconstruction of averaged ribosomes from Saccharomyces cerevisiae - Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
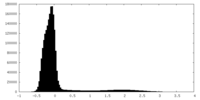
| Density Histograms |
- Sample components
Sample components
-Entire : Saccharomyces cerevisiae ribosomes
| Entire | Name: Saccharomyces cerevisiae ribosomes |
|---|---|
| Components |
|
-Supramolecule #1: Saccharomyces cerevisiae ribosomes
| Supramolecule | Name: Saccharomyces cerevisiae ribosomes / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Concentration: 200.0 mM / Component - Formula: CH3COONH4 / Component - Name: Ammoniumacetate Details: pH of the buffer was adjusted with NaOH buffer was filtered and sonicated |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.04 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: blot force 2 and blot time 6 before plunging. |
| Details | heterogenous cell extract |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Temperature | Min: 77.0 K / Max: 118.0 K |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 7 / Number real images: 2728 / Average exposure time: 3.61 sec. / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 44067 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 45000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)