+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | PTX3 Pentraxin Domain | |||||||||
 Map data Map data | Cryo electron microscopy derived map of the long pentraxin 3. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology information(1->3)-beta-D-glucan binding / negative regulation by host of viral exo-alpha-sialidase activity / negative regulation by host of viral glycoprotein metabolic process / negative regulation of exo-alpha-sialidase activity / negative regulation of glycoprotein metabolic process / ovarian cumulus expansion / negative regulation by host of viral process / opsonization / complement component C1q complex binding / response to yeast ...(1->3)-beta-D-glucan binding / negative regulation by host of viral exo-alpha-sialidase activity / negative regulation by host of viral glycoprotein metabolic process / negative regulation of exo-alpha-sialidase activity / negative regulation of glycoprotein metabolic process / ovarian cumulus expansion / negative regulation by host of viral process / opsonization / complement component C1q complex binding / response to yeast / negative regulation of viral entry into host cell / virion binding / positive regulation of phagocytosis / extracellular matrix organization / extracellular matrix / specific granule lumen / positive regulation of nitric oxide biosynthetic process / tertiary granule lumen / inflammatory response / innate immune response / Neutrophil degranulation / extracellular space / extracellular region / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
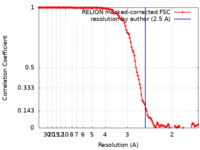
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Noone DP / Sharp TH | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: PTX3 structure determination using a hybrid cryoelectron microscopy and AlphaFold approach offers insights into ligand binding and complement activation. Authors: Dylan P Noone / Douwe J Dijkstra / Teun T van der Klugt / Peter A van Veelen / Arnoud H de Ru / Paul J Hensbergen / Leendert A Trouw / Thomas H Sharp /  Abstract: Pattern recognition molecules (PRMs) form an important part of innate immunity, where they facilitate the response to infections and damage by triggering processes such as inflammation. The pentraxin ...Pattern recognition molecules (PRMs) form an important part of innate immunity, where they facilitate the response to infections and damage by triggering processes such as inflammation. The pentraxin family of soluble PRMs comprises long and short pentraxins, with the former containing unique N-terminal regions unrelated to other proteins or each other. No complete high-resolution structural information exists about long pentraxins, unlike the short pentraxins, where there is an abundance of both X-ray and cryoelectron microscopy (cryo-EM)-derived structures. This study presents a high-resolution structure of the prototypical long pentraxin, PTX3. Cryo-EM yielded a 2.5-Å map of the C-terminal pentraxin domains that revealed a radically different quaternary structure compared to other pentraxins, comprising a glycosylated D4 symmetrical octameric complex stabilized by an extensive disulfide network. The cryo-EM map indicated α-helices that extended N terminal of the pentraxin domains that were not fully resolved. AlphaFold was used to predict the remaining N-terminal structure of the octameric PTX3 complex, revealing two long tetrameric coiled coils with two hinge regions, which was validated using classification of cryo-EM two-dimensional averages. The resulting hybrid cryo-EM/AlphaFold structure allowed mapping of ligand binding sites, such as C1q and fibroblast growth factor-2, as well as rationalization of previous biochemical data. Given the relevance of PTX3 in conditions ranging from COVID-19 prognosis, cancer progression, and female infertility, this structure could be used to inform the understanding and rational design of therapies for these disorders and processes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14774.map.gz emd_14774.map.gz | 12.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14774-v30.xml emd-14774-v30.xml emd-14774.xml emd-14774.xml | 21.7 KB 21.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14774_fsc.xml emd_14774_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_14774.png emd_14774.png | 134.2 KB | ||
| Masks |  emd_14774_msk_1.map emd_14774_msk_1.map | 178 MB |  Mask map Mask map | |
| Others |  emd_14774_additional_1.map.gz emd_14774_additional_1.map.gz emd_14774_half_map_1.map.gz emd_14774_half_map_1.map.gz emd_14774_half_map_2.map.gz emd_14774_half_map_2.map.gz | 166.1 MB 135.3 MB 135.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14774 http://ftp.pdbj.org/pub/emdb/structures/EMD-14774 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14774 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14774 | HTTPS FTP |
-Validation report
| Summary document |  emd_14774_validation.pdf.gz emd_14774_validation.pdf.gz | 619.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14774_full_validation.pdf.gz emd_14774_full_validation.pdf.gz | 619.3 KB | Display | |
| Data in XML |  emd_14774_validation.xml.gz emd_14774_validation.xml.gz | 19.8 KB | Display | |
| Data in CIF |  emd_14774_validation.cif.gz emd_14774_validation.cif.gz | 25.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14774 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14774 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14774 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14774 | HTTPS FTP |
-Related structure data
| Related structure data |  7zl1MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14774.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14774.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo electron microscopy derived map of the long pentraxin 3. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.836 Å | ||||||||||||||||||||
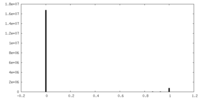
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_14774_msk_1.map emd_14774_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
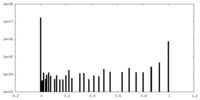
| Density Histograms |
-Additional map: Unmasked version of the map of the long pentraxin 3.
| File | emd_14774_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unmasked version of the map of the long pentraxin 3. | ||||||||||||
| Projections & Slices |
| ||||||||||||
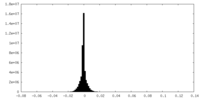
| Density Histograms |
-Half map: Half map of the refinement job in Relion.
| File | emd_14774_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the refinement job in Relion. | ||||||||||||
| Projections & Slices |
| ||||||||||||
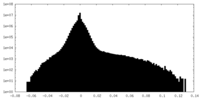
| Density Histograms |
-Half map: Half map of the refinement job in Relion.
| File | emd_14774_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the refinement job in Relion. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Long pentraxin 3 pentraxin domain
| Entire | Name: Long pentraxin 3 pentraxin domain |
|---|---|
| Components |
|
-Supramolecule #1: Long pentraxin 3 pentraxin domain
| Supramolecule | Name: Long pentraxin 3 pentraxin domain / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: CryoEM derived map of the pentraxin domains of PTX3. At higher isosurface threshold values the start of the coiled coil N-terminal domain can be seen. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 336 KDa |
-Macromolecule #1: Pentraxin-related protein PTX3
| Macromolecule | Name: Pentraxin-related protein PTX3 / type: protein_or_peptide / ID: 1 Details: Pentraxin domain of the long pentraxin 3. No N-terminal domain is included in this coordinate file. Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.842914 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: HHHHHHENLY FQGENSDDYD LMYVNLDNEI DNGLHPTEDP TPCACGQEHS EWDKLFIMLE NSQMRERMLL QATDDVLRGE LQRLREELG RLAESLARPC APGAPAEARL TSALDELLQA TRDAGRRLAR MEGAEAQRPE EAGRALAAVL EELRQTRADL H AVQGWAAR ...String: HHHHHHENLY FQGENSDDYD LMYVNLDNEI DNGLHPTEDP TPCACGQEHS EWDKLFIMLE NSQMRERMLL QATDDVLRGE LQRLREELG RLAESLARPC APGAPAEARL TSALDELLQA TRDAGRRLAR MEGAEAQRPE EAGRALAAVL EELRQTRADL H AVQGWAAR SWLPAGCETA ILFPMRSKKI FGSVHPVRPM RLESFSACIW VKATDVLNKT ILFSYGTKRN PYEIQLYLSY QS IVFVVGG EENKLVAEAM VSLGRWTHLC GTWNSEEGLT SLWVNGELAA TTVEMATGHI VPEGGILQIG QEKNGCCVGG GFD ETLAFS GRLTGFNIWD SVLSNEEIRE TGGAESCHIR GNIVGWGVTE IQPHGGAQYV S |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 8 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 379 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 23 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: GRAPHENE OXIDE / Support film - topology: HOLEY | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 65 % / Chamber temperature: 277.15 K / Instrument: LEICA EM GP | ||||||||||||
| Details | PTX3 at concentrations between 1-2 uM in 20 mM Tris-HCl, 500 mM NaCl, pH 8.0 was concentrated in a 50 kDa molecular weight cut-off spin filter to concentrations between 60-70 uM. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 90.15 K / Max: 103.15 K |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 4612 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z
Z Y
Y X
X