[English] 日本語
 Yorodumi
Yorodumi- EMDB-14459: P. berghei kinesin-8B motor domain in no nucleotide state bound t... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 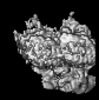 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | P. berghei kinesin-8B motor domain in no nucleotide state bound to tubulin dimer | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | kinesin / MOTOR PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationspindle elongation / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins ...spindle elongation / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Hedgehog 'off' state / Cilium Assembly / Intraflagellar transport / COPI-dependent Golgi-to-ER retrograde traffic / Carboxyterminal post-translational modifications of tubulin / RHOH GTPase cycle / Sealing of the nuclear envelope (NE) by ESCRT-III / Kinesins / PKR-mediated signaling / The role of GTSE1 in G2/M progression after G2 checkpoint / Aggrephagy / Resolution of Sister Chromatid Cohesion / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Separation of Sister Chromatids / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation / Recruitment of NuMA to mitotic centrosomes / microtubule associated complex / COPI-mediated anterograde transport / microtubule motor activity / microtubule-based movement / mitotic spindle organization / structural constituent of cytoskeleton / microtubule cytoskeleton organization / microtubule cytoskeleton / mitotic cell cycle / microtubule binding / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / microtubule / GTPase activity / GTP binding / ATP binding / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
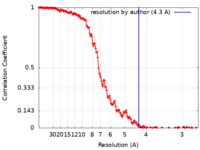
| Method | single particle reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Liu T / Shilliday F / Cook AD / Moores CA | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation | Journal: Protein Sci / Year: 2018 Title: UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Authors: Thomas D Goddard / Conrad C Huang / Elaine C Meng / Eric F Pettersen / Gregory S Couch / John H Morris / Thomas E Ferrin /  Abstract: UCSF ChimeraX is next-generation software for the visualization and analysis of molecular structures, density maps, 3D microscopy, and associated data. It addresses challenges in the size, scope, and ...UCSF ChimeraX is next-generation software for the visualization and analysis of molecular structures, density maps, 3D microscopy, and associated data. It addresses challenges in the size, scope, and disparate types of data attendant with cutting-edge experimental methods, while providing advanced options for high-quality rendering (interactive ambient occlusion, reliable molecular surface calculations, etc.) and professional approaches to software design and distribution. This article highlights some specific advances in the areas of visualization and usability, performance, and extensibility. ChimeraX is free for noncommercial use and is available from http://www.rbvi.ucsf.edu/chimerax/ for Windows, Mac, and Linux. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14459.map.gz emd_14459.map.gz | 472.9 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14459-v30.xml emd-14459-v30.xml emd-14459.xml emd-14459.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14459_fsc.xml emd_14459_fsc.xml | 19.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_14459.png emd_14459.png | 43.7 KB | ||
| Filedesc metadata |  emd-14459.cif.gz emd-14459.cif.gz | 6.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14459 http://ftp.pdbj.org/pub/emdb/structures/EMD-14459 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14459 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14459 | HTTPS FTP |
-Validation report
| Summary document |  emd_14459_validation.pdf.gz emd_14459_validation.pdf.gz | 418.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14459_full_validation.pdf.gz emd_14459_full_validation.pdf.gz | 418 KB | Display | |
| Data in XML |  emd_14459_validation.xml.gz emd_14459_validation.xml.gz | 11.3 KB | Display | |
| Data in CIF |  emd_14459_validation.cif.gz emd_14459_validation.cif.gz | 16.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14459 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14459 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14459 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14459 | HTTPS FTP |
-Related structure data
| Related structure data |  7z2aMC  7z2bC  7z2cC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14459.map.gz / Format: CCP4 / Size: 2.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14459.map.gz / Format: CCP4 / Size: 2.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : P. berghei kinesin-8B motor domain in no nucleotide state bound t...
| Entire | Name: P. berghei kinesin-8B motor domain in no nucleotide state bound to tubulin dimer |
|---|---|
| Components |
|
-Supramolecule #1: P. berghei kinesin-8B motor domain in no nucleotide state bound t...
| Supramolecule | Name: P. berghei kinesin-8B motor domain in no nucleotide state bound to tubulin dimer type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Detyrosinated tubulin alpha-1B chain
| Macromolecule | Name: Detyrosinated tubulin alpha-1B chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.833184 KDa |
| Sequence | String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPDSF NTFFSETGAG KHVPRAVFVD LEPTVIDEVR TGTYRQLFHP EQLITGKED AANNYARGHY TIGKEIIDLV LDRIRKLADQ CTGLQGFLVF HSFGGGTGSG FTSLLMERLS VDYGKKSKLE F SIYPAPQV ...String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPDSF NTFFSETGAG KHVPRAVFVD LEPTVIDEVR TGTYRQLFHP EQLITGKED AANNYARGHY TIGKEIIDLV LDRIRKLADQ CTGLQGFLVF HSFGGGTGSG FTSLLMERLS VDYGKKSKLE F SIYPAPQV STAVVEPYNS ILTTHTTLEH SDCAFMVDNE AIYDICRRNL DIERPTYTNL NRLISQIVSS ITASLRFDGA LN VDLTEFQ TNLVPYPRIH FPLATYAPVI SAEKAYHEQL SVAEITNACF EPANQMVKCD PRHGKYMACC LLYRGDVVPK DVN AAIATI KTKRSIQFVD WCPTGFKVGI NYQPPTVVPG GDLAKVQRAV CMLSNTTAIA EAWARLDHKF DLMYAKRAFV HWYV GEGME EGEFSEARED MAALEKDYEE VGV UniProtKB: Tubulin alpha-1B chain |
-Macromolecule #2: Tubulin beta chain
| Macromolecule | Name: Tubulin beta chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.825859 KDa |
| Sequence | String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGSYHGDS DLQLERINVY YNEAAGNKYV PRAILVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKESESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS ...String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGSYHGDS DLQLERINVY YNEAAGNKYV PRAILVDLEP GTMDSVRSGP FGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV VRKESESCDC LQGFQLTHSL GGGTGSGMGT LLISKIREEY P DRIMNTFS VVPSPKVSDT VVEPYNATLS VHQLVENTDE TYCIDNEALY DICFRTLKLT TPTYGDLNHL VSATMSGVTT CL RFPGQLN ADLRKLAVNM VPFPRLHFFM PGFAPLTSRG SQQYRALTVP ELTQQMFDAK NMMAACDPRH GRYLTVAAVF RGR MSMKEV DEQMLNVQNK NSSYFVEWIP NNVKTAVCDI PPRGLKMSAT FIGNSTAIQE LFKRISEQFT AMFRRKAFLH WYTG EGMDE MEFTEAESNM NDLVSEYQQY Q UniProtKB: Tubulin beta chain |
-Macromolecule #3: Kinesin-8, putative
| Macromolecule | Name: Kinesin-8, putative / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.086586 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DITYNMNVVI RCRPMSNSEK NEGAKNVIKI MDNKMIVLLD PSDNTDNVLR QNRTKEKRYC FDYVFDENST QEDVYNNSVK PLVDAVIKG YNSTVFAYGA TGAGKTHTII GYKNEPGIMM MILQDLFKKI KTLKAMNEYK IKCSFIEIYN ENICDLLNPS S EYLDLRED ...String: DITYNMNVVI RCRPMSNSEK NEGAKNVIKI MDNKMIVLLD PSDNTDNVLR QNRTKEKRYC FDYVFDENST QEDVYNNSVK PLVDAVIKG YNSTVFAYGA TGAGKTHTII GYKNEPGIMM MILQDLFKKI KTLKAMNEYK IKCSFIEIYN ENICDLLNPS S EYLDLRED PVKGITVSNI FEVCTTSVEE IMELIHTGNR NRTQEPTDAN RTSSRSHGVL QVIVEETEKG QGLYQQTKKG KL CVIDLAG SERASQTNNK GMRMLEGANI NRSLLALGNV INALVSRSKG TSKSNFIPFR DSKLTRLLKD SLGGNCKTLM IAN ISPSHL SYEDTHNTLK YANRAKNIKN V UniProtKB: Kinesin-8, putative |
-Macromolecule #4: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 1 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER
| Macromolecule | Name: PHOSPHOMETHYLPHOSPHONIC ACID GUANYLATE ESTER / type: ligand / ID: 6 / Number of copies: 1 / Formula: G2P |
|---|---|
| Molecular weight | Theoretical: 521.208 Da |
| Chemical component information |  ChemComp-G2P: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 6.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 50.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.5 µm / Calibrated defocus min: 0.5 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7z2a: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)