+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Outward-facing apo-form of auxin transporter PIN8 in detergent | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Auxin transport / AEC family / BART superfamily / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationauxin export across the plasma membrane / pollen development / auxin-activated signaling pathway / endoplasmic reticulum membrane / endoplasmic reticulum / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
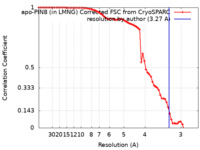
| Method | single particle reconstruction / cryo EM / Resolution: 3.27 Å | |||||||||
 Authors Authors | Ung KL / Winkler MBL / Dedic E / Stokes DL / Pedersen BP | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structures and mechanism of the plant PIN-FORMED auxin transporter. Authors: Kien Lam Ung / Mikael Winkler / Lukas Schulz / Martina Kolb / Dorina P Janacek / Emil Dedic / David L Stokes / Ulrich Z Hammes / Bjørn Panyella Pedersen /    Abstract: Auxins are hormones that have central roles and control nearly all aspects of growth and development in plants. The proteins in the PIN-FORMED (PIN) family (also known as the auxin efflux carrier ...Auxins are hormones that have central roles and control nearly all aspects of growth and development in plants. The proteins in the PIN-FORMED (PIN) family (also known as the auxin efflux carrier family) are key participants in this process and control auxin export from the cytosol to the extracellular space. Owing to a lack of structural and biochemical data, the molecular mechanism of PIN-mediated auxin transport is not understood. Here we present biophysical analysis together with three structures of Arabidopsis thaliana PIN8: two outward-facing conformations with and without auxin, and one inward-facing conformation bound to the herbicide naphthylphthalamic acid. The structure forms a homodimer, with each monomer divided into a transport and scaffold domain with a clearly defined auxin binding site. Next to the binding site, a proline-proline crossover is a pivot point for structural changes associated with transport, which we show to be independent of proton and ion gradients and probably driven by the negative charge of the auxin. The structures and biochemical data reveal an elevator-type transport mechanism reminiscent of bile acid/sodium symporters, bicarbonate/sodium symporters and sodium/proton antiporters. Our results provide a comprehensive molecular model for auxin recognition and transport by PINs, link and expand on a well-known conceptual framework for transport, and explain a central mechanism of polar auxin transport, a core feature of plant physiology, growth and development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14118.map.gz emd_14118.map.gz | 32.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14118-v30.xml emd-14118-v30.xml emd-14118.xml emd-14118.xml | 18.7 KB 18.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14118_fsc.xml emd_14118_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_14118.png emd_14118.png | 118 KB | ||
| Masks |  emd_14118_msk_1.map emd_14118_msk_1.map | 34.3 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-14118.cif.gz emd-14118.cif.gz | 5.9 KB | ||
| Others |  emd_14118_half_map_1.map.gz emd_14118_half_map_1.map.gz emd_14118_half_map_2.map.gz emd_14118_half_map_2.map.gz | 31.7 MB 31.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14118 http://ftp.pdbj.org/pub/emdb/structures/EMD-14118 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14118 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14118 | HTTPS FTP |
-Validation report
| Summary document |  emd_14118_validation.pdf.gz emd_14118_validation.pdf.gz | 806.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14118_full_validation.pdf.gz emd_14118_full_validation.pdf.gz | 806.2 KB | Display | |
| Data in XML |  emd_14118_validation.xml.gz emd_14118_validation.xml.gz | 14.3 KB | Display | |
| Data in CIF |  emd_14118_validation.cif.gz emd_14118_validation.cif.gz | 18.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14118 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14118 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14118 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14118 | HTTPS FTP |
-Related structure data
| Related structure data |  7qp9C  7qpaC  7qpcC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14118.map.gz / Format: CCP4 / Size: 34.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14118.map.gz / Format: CCP4 / Size: 34.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.306 Å | ||||||||||||||||||||||||||||||||||||
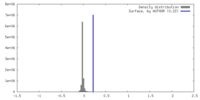
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_14118_msk_1.map emd_14118_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
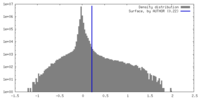
| Density Histograms |
-Half map: #1
| File | emd_14118_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_14118_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Apo-form of PIN8
| Entire | Name: Apo-form of PIN8 |
|---|---|
| Components |
|
-Supramolecule #1: Apo-form of PIN8
| Supramolecule | Name: Apo-form of PIN8 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: dimer form of PIN8 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 81 KDa |
-Macromolecule #1: Auxin efflux carrier component 8 (PIN-FORMED auxin transporter 8)
| Macromolecule | Name: Auxin efflux carrier component 8 (PIN-FORMED auxin transporter 8) type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MGISWLDIYH VVSATVPLYV SMTLGFLSAR HLKLFSPEQC AGINKFVAKF SIPLLSFQII SENNPFKMSP KLILSDILQK FLVVVVLAMV LRFWHPTGGR GGKLGWVITG LSISVLPNTL ILGMPILSAI YGDEAASILE QIVVLQSLIW YTILLFLFEL NAARALPSSG ...String: MGISWLDIYH VVSATVPLYV SMTLGFLSAR HLKLFSPEQC AGINKFVAKF SIPLLSFQII SENNPFKMSP KLILSDILQK FLVVVVLAMV LRFWHPTGGR GGKLGWVITG LSISVLPNTL ILGMPILSAI YGDEAASILE QIVVLQSLIW YTILLFLFEL NAARALPSSG ASLEHTGNDQ EEANIEDEPK EEEDEEEVAI VRTRSVGTMK ILLKAWRKLI INPNTYATLI GIIWATLHFR LGWNLPEMID KSIHLLSDGG LGMAMFSLGL FMASQSSIIA CGTKMAIITM LLKFVLGPAL MIASAYCIRL KSTLFKVAIL QAALPQGVVP FVFAKEYNLH PEIISTGVIF GMLIALPTTL AYYFLLDLPG ENLYFQ UniProtKB: Auxin efflux carrier component 8 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 20 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Details: The grid was glow-discharge at 15 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: Wait 4 seconds after sample loading, Blotting time 4 seconds with blotting force of -1 before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 7808 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)