+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
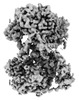
| Title | Mitochondrial DNA dependent RNA polymerase homodimer. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationMitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial promoter sequence-specific DNA binding / transcription initiation at mitochondrial promoter / mitochondrial transcription / DNA primase activity / mitochondrial nucleoid / Transcriptional activation of mitochondrial biogenesis / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase ...Mitochondrial transcription initiation / mitochondrial DNA-directed RNA polymerase complex / mitochondrial promoter sequence-specific DNA binding / transcription initiation at mitochondrial promoter / mitochondrial transcription / DNA primase activity / mitochondrial nucleoid / Transcriptional activation of mitochondrial biogenesis / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase / 3'-5'-RNA exonuclease activity / sequence-specific DNA binding / mitochondrial matrix / protein-containing complex / mitochondrion / RNA binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Das H / Hallberg BM | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Non-coding 7S RNA inhibits transcription via mitochondrial RNA polymerase dimerization. Authors: Xuefeng Zhu / Xie Xie / Hrishikesh Das / Benedict G Tan / Yonghong Shi / Ali Al-Behadili / Bradley Peter / Elisa Motori / Sebastian Valenzuela / Viktor Posse / Claes M Gustafsson / B Martin ...Authors: Xuefeng Zhu / Xie Xie / Hrishikesh Das / Benedict G Tan / Yonghong Shi / Ali Al-Behadili / Bradley Peter / Elisa Motori / Sebastian Valenzuela / Viktor Posse / Claes M Gustafsson / B Martin Hällberg / Maria Falkenberg /   Abstract: The mitochondrial genome encodes 13 components of the oxidative phosphorylation system, and altered mitochondrial transcription drives various human pathologies. A polyadenylated, non-coding RNA ...The mitochondrial genome encodes 13 components of the oxidative phosphorylation system, and altered mitochondrial transcription drives various human pathologies. A polyadenylated, non-coding RNA molecule known as 7S RNA is transcribed from a region immediately downstream of the light strand promoter in mammalian cells, and its levels change rapidly in response to physiological conditions. Here, we report that 7S RNA has a regulatory function, as it controls levels of mitochondrial transcription both in vitro and in cultured human cells. Using cryo-EM, we show that POLRMT dimerization is induced by interactions with 7S RNA. The resulting POLRMT dimer interface sequesters domains necessary for promoter recognition and unwinding, thereby preventing transcription initiation. We propose that the non-coding 7S RNA molecule is a component of a negative feedback loop that regulates mitochondrial transcription in mammalian cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13735.map.gz emd_13735.map.gz | 97 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13735-v30.xml emd-13735-v30.xml emd-13735.xml emd-13735.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_13735.png emd_13735.png | 95.2 KB | ||
| Others |  emd_13735_additional_1.map.gz emd_13735_additional_1.map.gz emd_13735_additional_2.map.gz emd_13735_additional_2.map.gz | 51.5 MB 51.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13735 http://ftp.pdbj.org/pub/emdb/structures/EMD-13735 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13735 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13735 | HTTPS FTP |
-Validation report
| Summary document |  emd_13735_validation.pdf.gz emd_13735_validation.pdf.gz | 457.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13735_full_validation.pdf.gz emd_13735_full_validation.pdf.gz | 457.3 KB | Display | |
| Data in XML |  emd_13735_validation.xml.gz emd_13735_validation.xml.gz | 6.3 KB | Display | |
| Data in CIF |  emd_13735_validation.cif.gz emd_13735_validation.cif.gz | 7.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13735 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13735 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13735 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13735 | HTTPS FTP |
-Related structure data
| Related structure data |  7pzpMC  7pzrC  7zc4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13735.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13735.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.01 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: localized reconstruction
| File | emd_13735_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | localized reconstruction | ||||||||||||
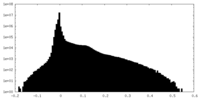
| Projections & Slices |
| ||||||||||||
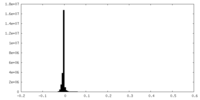
| Density Histograms |
-Additional map: localized reconstruction
| File | emd_13735_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | localized reconstruction | ||||||||||||
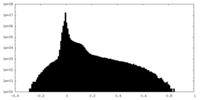
| Projections & Slices |
| ||||||||||||
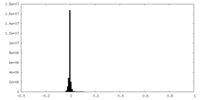
| Density Histograms |
- Sample components
Sample components
-Entire : POLRMT
| Entire | Name: POLRMT |
|---|---|
| Components |
|
-Supramolecule #1: POLRMT
| Supramolecule | Name: POLRMT / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: DNA-directed RNA polymerase, mitochondrial |
|---|---|
| Molecular weight | Theoretical: 137 KDa |
-Supramolecule #2: Mitochondrial DNA-directed RNA polymerase dimer.
| Supramolecule | Name: Mitochondrial DNA-directed RNA polymerase dimer. / type: complex / Chimera: Yes / ID: 2 / Parent: 1 / Macromolecule list: all / Details: DNA-directed RNA polymerase dimer, mitochondrial |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Macromolecule #1: DNA-directed RNA polymerase, mitochondrial
| Macromolecule | Name: DNA-directed RNA polymerase, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: DNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137.292125 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHTSG VDLGTENLYF QSSSASPQEQ DQDRRKDWGH VELLEVLQAR VRQLQAESVS EVVVNRVDVA RLPECGSGDG SLQPPRKVQ MGAKDATPVP CGRWAKILEK DKRTQQMRMQ RLKAKLQMPF QSGEFKALTR RLQVEPRLLS KQMAGCLEDC T RQAPESPW ...String: MHHHHHHTSG VDLGTENLYF QSSSASPQEQ DQDRRKDWGH VELLEVLQAR VRQLQAESVS EVVVNRVDVA RLPECGSGDG SLQPPRKVQ MGAKDATPVP CGRWAKILEK DKRTQQMRMQ RLKAKLQMPF QSGEFKALTR RLQVEPRLLS KQMAGCLEDC T RQAPESPW EEQLARLLQE APGKLSLDVE QAPSGQHSQA QLSGQQQRLL AFFKCCLLTD QLPLAHHLLV VHHGQRQKRK LL TLDMYNA VMLGWARQGA FKELVYVLFM VKDAGLTPDL LSYAAALQCM GRQDQDAGTI ERCLEQMSQE GLKLQALFTA VLL SEEDRA TVLKAVHKVK PTFSLPPQLP PPVNTSKLLR DVYAKDGRVS YPKLHLPLKT LQCLFEKQLH MELASRVCVV SVEK PTLPS KEVKHARKTL KTLRDQWEKA LCRALRETKN RLEREVYEGR FSLYPFLCLL DEREVVRMLL QVLQALPAQG ESFTT LARE LSARTFSRHV VQRQRVSGQV QALQNHYRKY LCLLASDAEV PEPCLPRQYW EELGAPEALR EQPWPLPVQM ELGKLL AEM LVQATQMPCS LDKPHRSSRL VPVLYHVYSF RNVQQIGILK PHPAYVQLLE KAAEPTLTFE AVDVPMLCPP LPWTSPH SG AFLLSPTKLM RTVEGATQHQ ELLETCPPTA LHGALDALTQ LGNCAWRVNG RVLDLVLQLF QAKGCPQLGV PAPPSEAP Q PPEAHLPHSA APARKAELRR ELAHCQKVAR EMHSLRAEAL YRLSLAQHLR DRVFWLPHNM DFRGRTYPCP PHFNHLGSD VARALLEFAQ GRPLGPHGLD WLKIHLVNLT GLKKREPLRK RLAFAEEVMD DILDSADQPL TGRKWWMGAE EPWQTLACCM EVANAVRAS DPAAYVSHLP VHQDGSCNGL QHYAALGRDS VGAASVNLEP SDVPQDVYSG VAAQVEVFRR QDAQRGMRVA Q VLEGFITR KVVKQTVMTV VYGVTRYGGR LQIEKRLREL SDFPQEFVWE ASHYLVRQVF KSLQEMFSGT RAIQHWLTES AR LISHMGS VVEWVTPLGV PVIQPYRLDS KVKQIGGGIQ SITYTHNGDI SRKPNTRKQK NGFPPNFIHS LDSSHMMLTA LHC YRKGLT FVSVHDCYWT HAADVSVMNQ VCREQFVRLH SEPILQDLSR FLVKRFCSEP QKILEASQLK ETLQAVPKPG AFDL EQVKR STYFFS |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.1 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 5 / Number real images: 42131 / Average exposure time: 1.5 sec. / Average electron dose: 48.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 20.0 µm / Calibrated defocus min: 0.3 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.3 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z
Z Y
Y X
X