+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13701 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Ca2+ free Drosophila Slo channel | |||||||||
 Map data Map data | Density modified using Phenix.Resolve | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Potassium transport / BK channel / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationSperm Motility And Taxes / negative regulation of neuromuscular synaptic transmission / male courtship behavior, veined wing generated song production / regulation of synaptic assembly at neuromuscular junction / large conductance calcium-activated potassium channel activity / calcium-activated potassium channel activity / circadian behavior / monoatomic ion channel complex / potassium ion transmembrane transport / potassium ion transport ...Sperm Motility And Taxes / negative regulation of neuromuscular synaptic transmission / male courtship behavior, veined wing generated song production / regulation of synaptic assembly at neuromuscular junction / large conductance calcium-activated potassium channel activity / calcium-activated potassium channel activity / circadian behavior / monoatomic ion channel complex / potassium ion transmembrane transport / potassium ion transport / circadian rhythm / postsynaptic membrane / neuron projection / response to xenobiotic stimulus / neuronal cell body / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
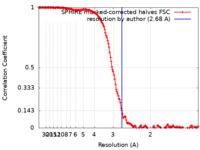
| Method | single particle reconstruction / cryo EM / Resolution: 2.68 Å | |||||||||
 Authors Authors | Raisch T / Brockmann A | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Small molecule modulation of the Drosophila Slo channel elucidated by cryo-EM. Authors: Tobias Raisch / Andreas Brockmann / Ulrich Ebbinghaus-Kintscher / Jörg Freigang / Oliver Gutbrod / Jan Kubicek / Barbara Maertens / Oliver Hofnagel / Stefan Raunser /  Abstract: Slowpoke (Slo) potassium channels display extraordinarily high conductance, are synergistically activated by a positive transmembrane potential and high intracellular Ca concentrations and are ...Slowpoke (Slo) potassium channels display extraordinarily high conductance, are synergistically activated by a positive transmembrane potential and high intracellular Ca concentrations and are important targets for insecticides and antiparasitic drugs. However, it is unknown how these compounds modulate ion translocation and whether there are insect-specific binding pockets. Here, we report structures of Drosophila Slo in the Ca-bound and Ca-free form and in complex with the fungal neurotoxin verruculogen and the anthelmintic drug emodepside. Whereas the architecture and gating mechanism of Slo channels are conserved, potential insect-specific binding pockets exist. Verruculogen inhibits K transport by blocking the Ca-induced activation signal and precludes K from entering the selectivity filter. Emodepside decreases the conductance by suboptimal K coordination and uncouples ion gating from Ca and voltage sensing. Our results expand the mechanistic understanding of Slo regulation and lay the foundation for the rational design of regulators of Slo and other voltage-gated ion channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13701.map.gz emd_13701.map.gz | 310.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13701-v30.xml emd-13701-v30.xml emd-13701.xml emd-13701.xml | 18.5 KB 18.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13701_fsc.xml emd_13701_fsc.xml | 17.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_13701.png emd_13701.png | 213.2 KB | ||
| Filedesc metadata |  emd-13701.cif.gz emd-13701.cif.gz | 6.2 KB | ||
| Others |  emd_13701_additional_1.map.gz emd_13701_additional_1.map.gz emd_13701_half_map_1.map.gz emd_13701_half_map_1.map.gz emd_13701_half_map_2.map.gz emd_13701_half_map_2.map.gz | 23 MB 165.5 MB 164.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13701 http://ftp.pdbj.org/pub/emdb/structures/EMD-13701 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13701 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13701 | HTTPS FTP |
-Validation report
| Summary document |  emd_13701_validation.pdf.gz emd_13701_validation.pdf.gz | 969.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13701_full_validation.pdf.gz emd_13701_full_validation.pdf.gz | 968.9 KB | Display | |
| Data in XML |  emd_13701_validation.xml.gz emd_13701_validation.xml.gz | 23.6 KB | Display | |
| Data in CIF |  emd_13701_validation.cif.gz emd_13701_validation.cif.gz | 31.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13701 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13701 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13701 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13701 | HTTPS FTP |
-Related structure data
| Related structure data |  7pxfMC  7pxeC  7pxgC  7pxhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13701.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13701.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Density modified using Phenix.Resolve | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
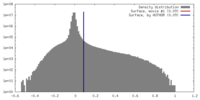
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Sharpened using SPHIRE
| File | emd_13701_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened using SPHIRE | ||||||||||||
| Projections & Slices |
| ||||||||||||
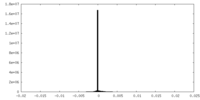
| Density Histograms |
-Half map: #2
| File | emd_13701_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
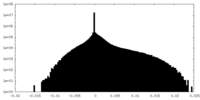
| Density Histograms |
-Half map: #1
| File | emd_13701_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
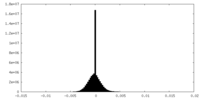
| Density Histograms |
- Sample components
Sample components
-Entire : Slo tetramer
| Entire | Name: Slo tetramer |
|---|---|
| Components |
|
-Supramolecule #1: Slo tetramer
| Supramolecule | Name: Slo tetramer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Isoform J of Calcium-activated potassium channel slowpoke
| Macromolecule | Name: Isoform J of Calcium-activated potassium channel slowpoke type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 130.919094 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASGLIDTNF SSTLANGMSG CDQSTVESLA DDPTDSPFDA DDCLKVRKYW CFLLSSIFTF LAGLLVVLLW RAFAFVCCRK EPDLGPNDP KQKEQKASRN KQEFEGTFMT EAKDWAGELI SGQTTTGRIL VVLVFILSIA SLIIYFVDAS SEEVERCQKW S NNITQQID ...String: MASGLIDTNF SSTLANGMSG CDQSTVESLA DDPTDSPFDA DDCLKVRKYW CFLLSSIFTF LAGLLVVLLW RAFAFVCCRK EPDLGPNDP KQKEQKASRN KQEFEGTFMT EAKDWAGELI SGQTTTGRIL VVLVFILSIA SLIIYFVDAS SEEVERCQKW S NNITQQID LAFNIFFMVY FFIRFIAASD KLWFMLEMYS FVDYFTIPPS FVSIYLDRTW IGLRFLRALR LMTVPDILQY LN VLKTSSS IRLAQLVSIF ISVWLTAAGI IHLLENSGDP LDFDNAHRLS YWTCVYFLIV TMSTVGYGDV YCETVLGRTF LVF FLLVGL AIFASCIPEI IDLIGTRAKY GGTLKNEKGR RHIVVCGHIT YESVSHFLKD FLHEDREDVD VEVVFLHRKP PDLE LEGLF KRHFTTVEFF QGTIMNPIDL QRVKVHEADA CLVLANKYCQ DPDAEDAANI MRVISIKNYS DDIRVIIQLM QYHNK AYLL NIPSWDWKQG DDVICLAELK LGFIAQSCLA PGFSTMMANL FAMRSFKTSP DMQSWTNDYL RGTGMEMYTE TLSPTF IGI PFAQATELCF SKLKLLLLAI EIKGAEEGAD SKISINPRGA KIQANTQGFF IAQSADEVKR AWFYCKACHE DIKDETL IK KCKCKNLATF RKGVRAVQMV GRASDITRDR EDTNLLNRNV RRPNGTGNGT GGMHHMNNTA AAAAAAAAAG KQVNKVKP T VNVSRQVEGQ VISPSQYNRP TSRSSGTGTQ NQNGGVSLPA GIADDQSKDF DFEKTEMKYD STGMFHWSPA KSLEDCILD RNQAAMTVLN GHVVVCLFAD PDSPLIGLRN LVMPLRASNF HYHELKHVVI VGSVDYIRRE WKMLQNLPKI SVLNGSPLSR ADLRAVNVN LCDMCCILSA KVPSNDDPTL ADKEAILASL NIKAMTFDDT IGVLSQRGPE FDNLSATAGS PIVLQRRGSV Y GANVPMIT ELVNDGNVQF LDQDDDDDPD TELYLTQPFA CGTAFAVSVL DSLMSTTYFN QNALTLIRSL ITGGATPELE LI LAEGAGL RGGYSTVESL SNRDRCRVGQ ISLYDGPLAQ FGECGKYGDL FVAALKSYGM LCIGLYRFRD TSSSCDASSK RYV ITNPPD DFSLLPTDQV FVLMQFDPGL EYKPPAVRAP AGGRGTNTQG SGVGGGGSNK DDNS UniProtKB: Calcium-activated potassium channel slowpoke |
-Macromolecule #2: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 2 / Number of copies: 4 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #3: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 3 / Number of copies: 3 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 79.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)