[English] 日本語
 Yorodumi
Yorodumi- EMDB-13552: Assembly intermediate of mouse mitochondrial ribosome small subun... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Assembly intermediate of mouse mitochondrial ribosome small subunit without mS37 in complex with RbfA inward conformation | ||||||||||||
 Map data Map data | Oversampled combined map of local-masked refined maps with sharpening and local-resolution filtering | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Assembly intermediate / maturation / biogenesis / small subunit / mitochondrion / RIBOSOME | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationMitochondrial translation elongation / Mitochondrial translation termination / mitochondrial ribosome binding / anaphase-promoting complex / mitochondrial ribosome assembly / positive regulation of mitochondrial translation / Mitochondrial protein degradation / mitochondrial ribosome / mitochondrial small ribosomal subunit / mitochondrial translation ...Mitochondrial translation elongation / Mitochondrial translation termination / mitochondrial ribosome binding / anaphase-promoting complex / mitochondrial ribosome assembly / positive regulation of mitochondrial translation / Mitochondrial protein degradation / mitochondrial ribosome / mitochondrial small ribosomal subunit / mitochondrial translation / positive regulation of proteolysis / apoptotic mitochondrial changes / ribosomal small subunit binding / rRNA processing / small ribosomal subunit rRNA binding / cell junction / regulation of translation / ribosomal small subunit assembly / nuclear membrane / cell population proliferation / tRNA binding / mitochondrial inner membrane / rRNA binding / ribosome / protein ubiquitination / structural constituent of ribosome / mitochondrial matrix / translation / protein domain specific binding / mRNA binding / nucleolus / mitochondrion / RNA binding / nucleoplasm / nucleus / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
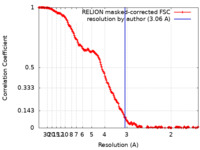
| Method | single particle reconstruction / cryo EM / Resolution: 3.06 Å | ||||||||||||
 Authors Authors | Itoh Y / Khawaja A | ||||||||||||
| Funding support | European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Mechanism of mitoribosomal small subunit biogenesis and preinitiation. Authors: Yuzuru Itoh / Anas Khawaja / Ivan Laptev / Miriam Cipullo / Ilian Atanassov / Petr Sergiev / Joanna Rorbach / Alexey Amunts /    Abstract: Mitoribosomes are essential for the synthesis and maintenance of bioenergetic proteins. Here we use cryo-electron microscopy to determine a series of the small mitoribosomal subunit (SSU) ...Mitoribosomes are essential for the synthesis and maintenance of bioenergetic proteins. Here we use cryo-electron microscopy to determine a series of the small mitoribosomal subunit (SSU) intermediates in complex with auxiliary factors, revealing a sequential assembly mechanism. The methyltransferase TFB1M binds to partially unfolded rRNA h45 that is promoted by RBFA, while the mRNA channel is blocked. This enables binding of METTL15 that promotes further rRNA maturation and a large conformational change of RBFA. The new conformation allows initiation factor mtIF3 to already occupy the subunit interface during the assembly. Finally, the mitochondria-specific ribosomal protein mS37 (ref. ) outcompetes RBFA to complete the assembly with the SSU-mS37-mtIF3 complex that proceeds towards mtIF2 binding and translation initiation. Our results explain how the action of step-specific factors modulate the dynamic assembly of the SSU, and adaptation of a unique protein, mS37, links the assembly to initiation to establish the catalytic human mitoribosome. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13552.map.gz emd_13552.map.gz | 651.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13552-v30.xml emd-13552-v30.xml emd-13552.xml emd-13552.xml | 56.5 KB 56.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13552_fsc.xml emd_13552_fsc.xml | 17.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13552.png emd_13552.png | 82.6 KB | ||
| Masks |  emd_13552_msk_1.map emd_13552_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13552.cif.gz emd-13552.cif.gz | 13.2 KB | ||
| Others |  emd_13552_additional_1.map.gz emd_13552_additional_1.map.gz emd_13552_half_map_1.map.gz emd_13552_half_map_1.map.gz emd_13552_half_map_2.map.gz emd_13552_half_map_2.map.gz | 374.1 MB 337.6 MB 337.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13552 http://ftp.pdbj.org/pub/emdb/structures/EMD-13552 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13552 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13552 | HTTPS FTP |
-Validation report
| Summary document |  emd_13552_validation.pdf.gz emd_13552_validation.pdf.gz | 987.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13552_full_validation.pdf.gz emd_13552_full_validation.pdf.gz | 986.9 KB | Display | |
| Data in XML |  emd_13552_validation.xml.gz emd_13552_validation.xml.gz | 25.6 KB | Display | |
| Data in CIF |  emd_13552_validation.cif.gz emd_13552_validation.cif.gz | 32.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13552 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13552 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13552 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13552 | HTTPS FTP |
-Related structure data
| Related structure data |  7pnuMC  7pntC  7pnvC  7pnwC  7pnxC  7pnyC  7pnzC  7po0C  7po1C  7po2C  7po3C  7po4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13552.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13552.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Oversampled combined map of local-masked refined maps with sharpening and local-resolution filtering | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.69167 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_13552_msk_1.map emd_13552_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Combined map of local-masked refined maps without sharpening
| File | emd_13552_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Combined map of local-masked refined maps without sharpening | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of the overall refinement
| File | emd_13552_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the overall refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of the overall refinement
| File | emd_13552_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of the overall refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Assembly intermediate of small subunit of human mitochondrial ribosome
+Supramolecule #1: Assembly intermediate of small subunit of human mitochondrial ribosome
+Macromolecule #1: 12S mitochondrial rRNA
+Macromolecule #2: 28S ribosomal protein S2, mitochondrial
+Macromolecule #3: 28S ribosomal protein S24, mitochondrial
+Macromolecule #4: 28S ribosomal protein S5, mitochondrial
+Macromolecule #5: 28S ribosomal protein S6, mitochondrial
+Macromolecule #6: 28S ribosomal protein S7, mitochondrial
+Macromolecule #7: 28S ribosomal protein S9, mitochondrial
+Macromolecule #8: 28S ribosomal protein S10, mitochondrial
+Macromolecule #9: 28S ribosomal protein S11, mitochondrial
+Macromolecule #10: 28S ribosomal protein S12, mitochondrial
+Macromolecule #11: 28S ribosomal protein S14, mitochondrial
+Macromolecule #12: 28S ribosomal protein S15, mitochondrial
+Macromolecule #13: 28S ribosomal protein S16, mitochondrial
+Macromolecule #14: 28S ribosomal protein S17, mitochondrial
+Macromolecule #15: 28S ribosomal protein S18b, mitochondrial
+Macromolecule #16: 28S ribosomal protein S18c, mitochondrial
+Macromolecule #17: 28S ribosomal protein S21, mitochondrial
+Macromolecule #18: 28S ribosomal protein S22, mitochondrial
+Macromolecule #19: 28S ribosomal protein S23, mitochondrial
+Macromolecule #20: 28S ribosomal protein S25, mitochondrial
+Macromolecule #21: 28S ribosomal protein S26, mitochondrial
+Macromolecule #22: 28S ribosomal protein S27, mitochondrial
+Macromolecule #23: 28S ribosomal protein S28, mitochondrial
+Macromolecule #24: 28S ribosomal protein S29, mitochondrial
+Macromolecule #25: 28S ribosomal protein S31, mitochondrial
+Macromolecule #26: 28S ribosomal protein S33, mitochondrial
+Macromolecule #27: 28S ribosomal protein S34, mitochondrial
+Macromolecule #28: 28S ribosomal protein S35, mitochondrial
+Macromolecule #29: Aurora kinase A-interacting protein
+Macromolecule #30: Pentatricopeptide repeat domain-containing protein 3, mitochondrial
+Macromolecule #31: Putative ribosome-binding factor A, mitochondrial
+Macromolecule #32: MAGNESIUM ION
+Macromolecule #33: POTASSIUM ION
+Macromolecule #34: ZINC ION
+Macromolecule #35: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #36: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #37: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 297 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-20 / Number real images: 26468 / Average exposure time: 4.0 sec. / Average electron dose: 31.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.6 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)