[English] 日本語
 Yorodumi
Yorodumi- EMDB-12533: Cryo-EM structure of the cytochrome bd oxidase from M. tuberculos... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12533 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the cytochrome bd oxidase from M. tuberculosis in presence of Aurachin D at 3.3 A resolution | |||||||||||||||
 Map data Map data | Postprocess map Applied b-factor: -127 | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationcytochrome complex / aerobic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / electron transfer activity / heme binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||||||||
| Biological species |  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) | |||||||||||||||
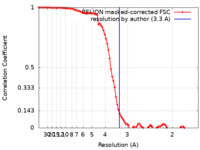
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||||||||
 Authors Authors | Safarian S / Wu D / Krause KL / Michel H | |||||||||||||||
| Funding support |  Germany, Germany,  New Zealand, 4 items New Zealand, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: The cryo-EM structure of the bd oxidase from M. tuberculosis reveals a unique structural framework and enables rational drug design to combat TB. Authors: Schara Safarian / Helen K Opel-Reading / Di Wu / Ahmad R Mehdipour / Kiel Hards / Liam K Harold / Melanie Radloff / Ian Stewart / Sonja Welsch / Gerhard Hummer / Gregory M Cook / Kurt L ...Authors: Schara Safarian / Helen K Opel-Reading / Di Wu / Ahmad R Mehdipour / Kiel Hards / Liam K Harold / Melanie Radloff / Ian Stewart / Sonja Welsch / Gerhard Hummer / Gregory M Cook / Kurt L Krause / Hartmut Michel /   Abstract: New drugs are urgently needed to combat the global TB epidemic. Targeting simultaneously multiple respiratory enzyme complexes of Mycobacterium tuberculosis is regarded as one of the most effective ...New drugs are urgently needed to combat the global TB epidemic. Targeting simultaneously multiple respiratory enzyme complexes of Mycobacterium tuberculosis is regarded as one of the most effective treatment options to shorten drug administration regimes, and reduce the opportunity for the emergence of drug resistance. During infection and proliferation, the cytochrome bd oxidase plays a crucial role for mycobacterial pathophysiology by maintaining aerobic respiration at limited oxygen concentrations. Here, we present the cryo-EM structure of the cytochrome bd oxidase from M. tuberculosis at 2.5 Å. In conjunction with atomistic molecular dynamics (MD) simulation studies we discovered a previously unknown MK-9-binding site, as well as a unique disulfide bond within the Q-loop domain that defines an inactive conformation of the canonical quinol oxidation site in Actinobacteria. Our detailed insights into the long-sought atomic framework of the cytochrome bd oxidase from M. tuberculosis will form the basis for the design of highly specific drugs to act on this enzyme. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12533.map.gz emd_12533.map.gz | 1.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12533-v30.xml emd-12533-v30.xml emd-12533.xml emd-12533.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12533_fsc.xml emd_12533_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_12533.png emd_12533.png | 139.3 KB | ||
| Others |  emd_12533_half_map_1.map.gz emd_12533_half_map_1.map.gz emd_12533_half_map_2.map.gz emd_12533_half_map_2.map.gz | 46.6 MB 46.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12533 http://ftp.pdbj.org/pub/emdb/structures/EMD-12533 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12533 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12533 | HTTPS FTP |
-Validation report
| Summary document |  emd_12533_validation.pdf.gz emd_12533_validation.pdf.gz | 343.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12533_full_validation.pdf.gz emd_12533_full_validation.pdf.gz | 342.5 KB | Display | |
| Data in XML |  emd_12533_validation.xml.gz emd_12533_validation.xml.gz | 15.8 KB | Display | |
| Data in CIF |  emd_12533_validation.cif.gz emd_12533_validation.cif.gz | 21.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12533 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12533 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12533 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12533 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12533.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12533.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocess map Applied b-factor: -127 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.837 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #1
| File | emd_12533_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_12533_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of the cytochrome bd oxidase from M. tuberculos...
| Entire | Name: Cryo-EM structure of the cytochrome bd oxidase from M. tuberculosis in presence of Aurachin D at 3.3 A resolution |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the cytochrome bd oxidase from M. tuberculos...
| Supramolecule | Name: Cryo-EM structure of the cytochrome bd oxidase from M. tuberculosis in presence of Aurachin D at 3.3 A resolution type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) / Recombinant plasmid: pYUB28b Mycolicibacterium smegmatis MC2 155 (bacteria) / Recombinant plasmid: pYUB28b |
| Molecular weight | Experimental: 91.5 KDa |
-Macromolecule #1: Probable integral membrane cytochrome D ubiquinol oxidase (Subuni...
| Macromolecule | Name: Probable integral membrane cytochrome D ubiquinol oxidase (Subunit II) CydB (Cytochrome BD-I oxidase subunit II) type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Sequence | String: MVLQELWFGV IAALFLGFFI LEGFDFGVGM LMAPFAHVGM GDPETHRRTA LNTIGPVWDG NEVWLITAGA AIFAAFPGWY ATVFSALYLP LLAILFGMIL RAVAIEWRGK IDDPKWRTGA DFGIAAGSWL PALLWGVAFA ILVRGLPVDA NGHVALSIPD VLNAYTLLGG ...String: MVLQELWFGV IAALFLGFFI LEGFDFGVGM LMAPFAHVGM GDPETHRRTA LNTIGPVWDG NEVWLITAGA AIFAAFPGWY ATVFSALYLP LLAILFGMIL RAVAIEWRGK IDDPKWRTGA DFGIAAGSWL PALLWGVAFA ILVRGLPVDA NGHVALSIPD VLNAYTLLGG LATAGLFSLY GAVFIALKTS GPIRDDAYRF AVWLSLPVAG LVAGFGLWTQ LAYGKDWTWL VLAVAGCAQA AATVLVWRRV SDGWAFMCTL IVVAAVVVLL FGALYPNLVP STLNPQWSLT IHNASSTPYT LKIMTWVTAF FAPLTVAYQT WTYWVFRQRI SAERIPPPTG LARRAP |
-Macromolecule #2: Probable integral membrane cytochrome D ubiquinol oxidase (Subuni...
| Macromolecule | Name: Probable integral membrane cytochrome D ubiquinol oxidase (Subunit I) CydA (Cytochrome BD-I oxidase subunit I) type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycobacterium tuberculosis H37Rv (bacteria) Mycobacterium tuberculosis H37Rv (bacteria) |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Sequence | String: MNVVDISRWQ FGITTVYHFI FVPLTIGLAP LIAVMQTLWV VTDNPAWYRL TKFFGKLFLI NFAIGVATGI VQEFQFGMNW SEYSRFVGDV FGAPLAMEGL AAFFFESTFI GLWIFGWNRL PRLVHLACIW IVAIAVNVSA FFIIAANSFM QHPVGAHYNP TTGRAELSSI ...String: MNVVDISRWQ FGITTVYHFI FVPLTIGLAP LIAVMQTLWV VTDNPAWYRL TKFFGKLFLI NFAIGVATGI VQEFQFGMNW SEYSRFVGDV FGAPLAMEGL AAFFFESTFI GLWIFGWNRL PRLVHLACIW IVAIAVNVSA FFIIAANSFM QHPVGAHYNP TTGRAELSSI VVLLTNNTAQ AAFTHTVSGA LLTAGTFVAA VSAWWLVRSS TTHADSDTQA MYRPATILGC WVALAATAGL LFTGDHQGKL MFQQQPMKMA SAESLCDTQT DPNFSVLTVG RQNNCDSLTR VIEVPYVLPF LAEGRISGVT LQGIRDLQQE YQQRFGPNDY RPNLFVTYWS FRMMIGLMAI PVLFALIALW LTRGGQIPNQ RWFSWLALLT MPAPFLANSA GWVFTEMGRQ PWVVVPNPTG DQLVRLTVKA GVSDHSATVV ATSLLMFTLV YAVLAVIWCW LLKRYIVEGP LEHDAEPAAH GAPRDDEVAP LSFAY |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 / Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Details: 15 mA | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: Blot force 20. | |||||||||
| Details | Monodisperse sample of cytochrome bd oxidase reconstituted in lipid nanodiscs 1D1 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 7401 / Average exposure time: 5.0 sec. / Average electron dose: 15.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 96000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 94 / Target criteria: Correlation coefficient |
|---|
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)