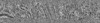[English] 日本語
 Yorodumi
Yorodumi- EMDB-12324: In situ cryo-electron tomogram of the Synechocystis wild-type VIP... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12324 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ cryo-electron tomogram of the Synechocystis wild-type VIPP1 strain grown in high light | |||||||||
 Map data Map data | Contrast-enhanced tomogram by deconvolution using the TOM software toolbox (TOMdeconv). This is a binned tomogram at 4x the original pixel size. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | electron tomography / cryo EM | |||||||||
 Authors Authors | Wietrzynski W / Klumpe S / Heinz S / Spaniol B / Schaffer M / Rast A / Nickelsen J / Schroda M / Engel BD | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: Structural basis for VIPP1 oligomerization and maintenance of thylakoid membrane integrity. Authors: Tilak Kumar Gupta / Sven Klumpe / Karin Gries / Steffen Heinz / Wojciech Wietrzynski / Norikazu Ohnishi / Justus Niemeyer / Benjamin Spaniol / Miroslava Schaffer / Anna Rast / Matthias ...Authors: Tilak Kumar Gupta / Sven Klumpe / Karin Gries / Steffen Heinz / Wojciech Wietrzynski / Norikazu Ohnishi / Justus Niemeyer / Benjamin Spaniol / Miroslava Schaffer / Anna Rast / Matthias Ostermeier / Mike Strauss / Jürgen M Plitzko / Wolfgang Baumeister / Till Rudack / Wataru Sakamoto / Jörg Nickelsen / Jan M Schuller / Michael Schroda / Benjamin D Engel /    Abstract: Vesicle-inducing protein in plastids 1 (VIPP1) is essential for the biogenesis and maintenance of thylakoid membranes, which transform light into life. However, it is unknown how VIPP1 performs its ...Vesicle-inducing protein in plastids 1 (VIPP1) is essential for the biogenesis and maintenance of thylakoid membranes, which transform light into life. However, it is unknown how VIPP1 performs its vital membrane-remodeling functions. Here, we use cryo-electron microscopy to determine structures of cyanobacterial VIPP1 rings, revealing how VIPP1 monomers flex and interweave to form basket-like assemblies of different symmetries. Three VIPP1 monomers together coordinate a non-canonical nucleotide binding pocket on one end of the ring. Inside the ring's lumen, amphipathic helices from each monomer align to form large hydrophobic columns, enabling VIPP1 to bind and curve membranes. In vivo mutations in these hydrophobic surfaces cause extreme thylakoid swelling under high light, indicating an essential role of VIPP1 lipid binding in resisting stress-induced damage. Using cryo-correlative light and electron microscopy (cryo-CLEM), we observe oligomeric VIPP1 coats encapsulating membrane tubules within the Chlamydomonas chloroplast. Our work provides a structural foundation for understanding how VIPP1 directs thylakoid biogenesis and maintenance. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12324.map.gz emd_12324.map.gz | 642.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12324-v30.xml emd-12324-v30.xml emd-12324.xml emd-12324.xml | 13.6 KB 13.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_12324.png emd_12324.png | 145.7 KB | ||
| Others |  emd_12324_additional_1.map.gz emd_12324_additional_1.map.gz | 640.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12324 http://ftp.pdbj.org/pub/emdb/structures/EMD-12324 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12324 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12324 | HTTPS FTP |
-Validation report
| Summary document |  emd_12324_validation.pdf.gz emd_12324_validation.pdf.gz | 204.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12324_full_validation.pdf.gz emd_12324_full_validation.pdf.gz | 203.7 KB | Display | |
| Data in XML |  emd_12324_validation.xml.gz emd_12324_validation.xml.gz | 4.6 KB | Display | |
| Data in CIF |  emd_12324_validation.cif.gz emd_12324_validation.cif.gz | 4.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12324 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12324 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12324 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12324 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12324.map.gz / Format: CCP4 / Size: 696.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12324.map.gz / Format: CCP4 / Size: 696.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Contrast-enhanced tomogram by deconvolution using the TOM software toolbox (TOMdeconv). This is a binned tomogram at 4x the original pixel size. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 14.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Original dose-filtered tomogram reconstruction. This is a binned...
| File | emd_12324_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Original dose-filtered tomogram reconstruction. This is a binned tomogram at 4x the original pixel size. | ||||||||||||
| Projections & Slices |
| ||||||||||||
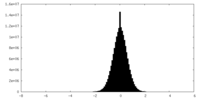
| Density Histograms |
- Sample components
Sample components
-Entire : Synechocystis wild-type VIPP1 strain grown in high light
| Entire | Name: Synechocystis wild-type VIPP1 strain grown in high light |
|---|---|
| Components |
|
-Supramolecule #1: Synechocystis wild-type VIPP1 strain grown in high light
| Supramolecule | Name: Synechocystis wild-type VIPP1 strain grown in high light type: cell / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron tomography |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE-PROPANE / Instrument: FEI VITROBOT MARK IV |
| Sectioning | Focused ion beam - Instrument: OTHER / Focused ion beam - Ion: OTHER / Focused ion beam - Voltage: 30 kV / Focused ion beam - Current: 0.03 nA / Focused ion beam - Duration: 1800 sec. / Focused ion beam - Temperature: 91 K / Focused ion beam - Initial thickness: 3000 nm / Focused ion beam - Final thickness: 200 nm Focused ion beam - Details: See https://bio-protocol.org/e1575 for detailed procedure.. The value given for _emd_sectioning_focused_ion_beam.instrument is FEI Aquilos FIB. This is not in a list of ...Focused ion beam - Details: See https://bio-protocol.org/e1575 for detailed procedure.. The value given for _emd_sectioning_focused_ion_beam.instrument is FEI Aquilos FIB. This is not in a list of allowed values {'OTHER', 'DB235'} so OTHER is written into the XML file. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Details | Dose-symmetric tilt-series were acquired (Hagen et al., 2017), starting at +10 degrees to match the pre-tilt of the lamella (i.e. from -50 to +70 degrees). |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 1.0 sec. / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.5 µm / Nominal defocus min: 4.0 µm / Nominal magnification: 42000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Algorithm: BACK PROJECTION / Software - Name:  IMOD / Number images used: 61 IMOD / Number images used: 61 |
|---|---|
| CTF correction | Software - Name:  IMOD IMOD |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)