[English] 日本語
 Yorodumi
Yorodumi- EMDB-11164: Amyloid fibril morphology II (ex vivo) from murine SAA1.1 protein. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11164 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Amyloid fibril morphology II (ex vivo) from murine SAA1.1 protein. | |||||||||||||||||||||
 Map data Map data | ex vivo mSAA amyloid fibril morphology II | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | systemic amyloidosis / misfolding disease /  inflammation / inflammation /  prion / PROTEIN FIBRIL prion / PROTEIN FIBRIL | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to stilbenoid / high-density lipoprotein particle / cytoplasmic microtubule / G protein-coupled receptor binding / acute-phase response Similarity search - Function | |||||||||||||||||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||||||||||||||
| Method | helical reconstruction /  cryo EM / Resolution: 3.5 Å cryo EM / Resolution: 3.5 Å | |||||||||||||||||||||
 Authors Authors | Bansal A / Schmidt M | |||||||||||||||||||||
| Funding support |  Germany, 6 items Germany, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: AA amyloid fibrils from diseased tissue are structurally different from in vitro formed SAA fibrils. Authors: Akanksha Bansal / Matthias Schmidt / Matthies Rennegarbe / Christian Haupt / Falk Liberta / Sabrina Stecher / Ioana Puscalau-Girtu / Alexander Biedermann / Marcus Fändrich /  Abstract: Systemic AA amyloidosis is a world-wide occurring protein misfolding disease of humans and animals. It arises from the formation of amyloid fibrils from serum amyloid A (SAA) protein. Using cryo ...Systemic AA amyloidosis is a world-wide occurring protein misfolding disease of humans and animals. It arises from the formation of amyloid fibrils from serum amyloid A (SAA) protein. Using cryo electron microscopy we here show that amyloid fibrils which were purified from AA amyloidotic mice are structurally different from fibrils formed from recombinant SAA protein in vitro. Ex vivo amyloid fibrils consist of fibril proteins that contain more residues within their ordered parts and possess a higher β-sheet content than in vitro fibril proteins. They are also more resistant to proteolysis than their in vitro formed counterparts. These data suggest that pathogenic amyloid fibrils may originate from proteolytic selection, allowing specific fibril morphologies to proliferate and to cause damage to the surrounding tissue. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11164.map.gz emd_11164.map.gz | 5.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11164-v30.xml emd-11164-v30.xml emd-11164.xml emd-11164.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11164.png emd_11164.png | 52.5 KB | ||
| Filedesc metadata |  emd-11164.cif.gz emd-11164.cif.gz | 5.1 KB | ||
| Others |  emd_11164_half_map_1.map.gz emd_11164_half_map_1.map.gz emd_11164_half_map_2.map.gz emd_11164_half_map_2.map.gz | 27 MB 27 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11164 http://ftp.pdbj.org/pub/emdb/structures/EMD-11164 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11164 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11164 | HTTPS FTP |
-Related structure data
| Related structure data |  6zchMC  6zcfC  6zcgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11164.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11164.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ex vivo mSAA amyloid fibril morphology II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: ex vivo mSAA amyloid fibril morphology II (half map II)
| File | emd_11164_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ex vivo mSAA amyloid fibril morphology II (half map II) | ||||||||||||
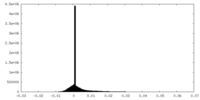
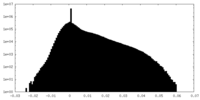
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: ex vivo mSAA amyloid fibril morphology II (half map I)
| File | emd_11164_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ex vivo mSAA amyloid fibril morphology II (half map I) | ||||||||||||
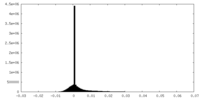
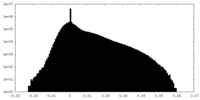
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Serum amyloid A1 (SAA1) amyloid fibril
| Entire | Name: Serum amyloid A1 (SAA1) amyloid fibril |
|---|---|
| Components |
|
-Supramolecule #1: Serum amyloid A1 (SAA1) amyloid fibril
| Supramolecule | Name: Serum amyloid A1 (SAA1) amyloid fibril / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: ex vivo murine SAA amyloid fibril morphology II |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
-Macromolecule #1: Serum amyloid A-2 protein
| Macromolecule | Name: Serum amyloid A-2 protein / type: protein_or_peptide / ID: 1 / Number of copies: 18 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 9.362094 KDa |
| Sequence | String: GFFSFIGEAF QGAGDMWRAY TDMKEAGWKD GDKYFHARGN YDAAQRGPGG VWAAEKISDA RESFQEFFGR GHEDTMADQE ANR UniProtKB:  Serum amyloid A-2 protein Serum amyloid A-2 protein |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7 / Details: water |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 96 % / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 20.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Segment selection | Number selected: 15530 |
|---|---|
| Startup model | Type of model: NONE |
| Final angle assignment | Type: NOT APPLICABLE |
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 4.80846 Å Applied symmetry - Helical parameters - Δ&Phi: -1.08807 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 2.1) / Number images used: 15505 |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: BACKBONE TRACE / Target criteria: Correlation coefficient |
|---|---|
| Output model |  PDB-6zch: |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X