[English] 日本語
 Yorodumi
Yorodumi- PDB-8sy5: E. coli DNA-directed RNA polymerase transcription elongation comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8sy5 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli DNA-directed RNA polymerase transcription elongation complex bound the unnatural dS-BTP base pair in the active site | |||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||
 Keywords Keywords | TRANSCRIPTION/DNA/RNA /  complex / unnatural base pairs / complex / unnatural base pairs /  synthetic biology / synthetic biology /  transcription / TRANSCRIPTION-DNA-RNA complex transcription / TRANSCRIPTION-DNA-RNA complex | |||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology information RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / transcription elongation factor complex / regulation of DNA-templated transcription elongation ... RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / transcription elongation factor complex / regulation of DNA-templated transcription elongation ... RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / transcription elongation factor complex / regulation of DNA-templated transcription elongation / transcription antitermination / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / bacterial-type flagellum assembly / bacterial-type flagellum-dependent cell motility / nitrate assimilation / transcription elongation factor complex / regulation of DNA-templated transcription elongation / transcription antitermination /  cell motility / DNA-templated transcription initiation / cell motility / DNA-templated transcription initiation /  ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity /  DNA-directed RNA polymerase / response to heat / protein-containing complex assembly / intracellular iron ion homeostasis / DNA-directed RNA polymerase / response to heat / protein-containing complex assembly / intracellular iron ion homeostasis /  protein dimerization activity / response to antibiotic / magnesium ion binding / protein dimerization activity / response to antibiotic / magnesium ion binding /  DNA binding / zinc ion binding / DNA binding / zinc ion binding /  membrane / membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||||||||||||||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli)synthetic construct (others) | |||||||||||||||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.7 Å cryo EM / Resolution: 2.7 Å | |||||||||||||||||||||||||||
 Authors Authors | Shan, Z. / Lyumkis, D. / Oh, J. / Wang, D. | |||||||||||||||||||||||||||
| Funding support |  United States, 8items United States, 8items
| |||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: A unified Watson-Crick geometry drives transcription of six-letter expanded DNA alphabets by E. coli RNA polymerase. Authors: Juntaek Oh / Zelin Shan / Shuichi Hoshika / Jun Xu / Jenny Chong / Steven A Benner / Dmitry Lyumkis / Dong Wang /   Abstract: Artificially Expanded Genetic Information Systems (AEGIS) add independently replicable unnatural nucleotide pairs to the natural G:C and A:T/U pairs found in native DNA, joining the unnatural pairs ...Artificially Expanded Genetic Information Systems (AEGIS) add independently replicable unnatural nucleotide pairs to the natural G:C and A:T/U pairs found in native DNA, joining the unnatural pairs through alternative modes of hydrogen bonding. Whether and how AEGIS pairs are recognized and processed by multi-subunit cellular RNA polymerases (RNAPs) remains unknown. Here, we show that E. coli RNAP selectively recognizes unnatural nucleobases in a six-letter expanded genetic system. High-resolution cryo-EM structures of three RNAP elongation complexes containing template-substrate UBPs reveal the shared principles behind the recognition of AEGIS and natural base pairs. In these structures, RNAPs are captured in an active state, poised to perform the chemistry step. At this point, the unnatural base pair adopts a Watson-Crick geometry, and the trigger loop is folded into an active conformation, indicating that the mechanistic principles underlying recognition and incorporation of natural base pairs also apply to AEGIS unnatural base pairs. These data validate the design philosophy of AEGIS unnatural basepairs. Further, we provide structural evidence supporting a long-standing hypothesis that pair mismatch during transcription occurs via tautomerization. Together, our work highlights the importance of Watson-Crick complementarity underlying the design principles of AEGIS base pair recognition. | |||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8sy5.cif.gz 8sy5.cif.gz | 518.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8sy5.ent.gz pdb8sy5.ent.gz | 393.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8sy5.json.gz 8sy5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sy/8sy5 https://data.pdbj.org/pub/pdb/validation_reports/sy/8sy5 ftp://data.pdbj.org/pub/pdb/validation_reports/sy/8sy5 ftp://data.pdbj.org/pub/pdb/validation_reports/sy/8sy5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  40862MC  8sy6C  8sy7C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-DNA-directed RNA polymerase subunit ... , 4 types, 5 molecules AGIJK
| #1: Protein |  Polymerase / RNAP subunit alpha / RNA polymerase subunit alpha / Transcriptase subunit alpha Polymerase / RNAP subunit alpha / RNA polymerase subunit alpha / Transcriptase subunit alphaMass: 36558.680 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Gene: rpoA, pez, phs, sez, b3295, JW3257 / Production host: Escherichia coli (E. coli) / Gene: rpoA, pez, phs, sez, b3295, JW3257 / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A7Z4, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A7Z4,  DNA-directed RNA polymerase DNA-directed RNA polymerase#2: Protein | |  Polymerase / RNAP subunit beta / RNA polymerase subunit beta / Transcriptase subunit beta Polymerase / RNAP subunit beta / RNA polymerase subunit beta / Transcriptase subunit betaMass: 150820.875 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) Escherichia coli (E. coli)Gene: rpoB, groN, nitB, rif, ron, stl, stv, tabD, b3987, JW3950 Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8V2, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8V2,  DNA-directed RNA polymerase DNA-directed RNA polymerase#3: Protein | |  Polymerase / RNAP subunit beta' / RNA polymerase subunit beta' / Transcriptase subunit beta' Polymerase / RNAP subunit beta' / RNA polymerase subunit beta' / Transcriptase subunit beta'Mass: 158105.672 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Gene: rpoC, tabB, b3988, JW3951 / Production host: Escherichia coli (E. coli) / Gene: rpoC, tabB, b3988, JW3951 / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8T7, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A8T7,  DNA-directed RNA polymerase DNA-directed RNA polymerase#4: Protein | |  Polymerase / RNAP omega subunit / RNA polymerase omega subunit / Transcriptase subunit omega Polymerase / RNAP omega subunit / RNA polymerase omega subunit / Transcriptase subunit omegaMass: 10249.547 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / Gene: rpoZ, b3649, JW3624 / Production host: Escherichia coli (E. coli) / Gene: rpoZ, b3649, JW3624 / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A800, Escherichia coli BL21(DE3) (bacteria) / References: UniProt: P0A800,  DNA-directed RNA polymerase DNA-directed RNA polymerase |
|---|
-DNA chain , 2 types, 2 molecules NT
| #5: DNA chain | Mass: 5663.694 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|---|
| #7: DNA chain | Mass: 8713.597 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
-RNA chain , 1 types, 1 molecules R
| #6: RNA chain | Mass: 2934.831 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|
-Non-polymers , 4 types, 379 molecules 

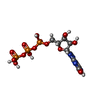




| #8: Chemical | | #9: Chemical | ChemComp-MG / | #10: Chemical | ChemComp-X0F / | #11: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: E. coli RNAP transcription elongation complex bound the unnatural dS-BTP base pair in the active site Type: COMPLEX / Entity ID: #1-#7 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 0.5 MDa / Experimental value: YES |
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Source (recombinant) | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
| Buffer solution | pH: 8 Details: 20mM Tris (pH 8.0), 40mM KCl, 5mM DTT and 5mM MgCl2 |
| Specimen | Conc.: 18 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YESDetails: The sample was concentrated to 18 mg/ml with 4 mM CHAPSO |
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: UltrAuFoil R1.2/1.3 |
Vitrification | Cryogen name: ETHANE / Humidity: 90 % |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Calibrated magnification: 58140 X / Nominal defocus max: 2600 nm / Nominal defocus min: 800 nm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE Bright-field microscopy / Calibrated magnification: 58140 X / Nominal defocus max: 2600 nm / Nominal defocus min: 800 nm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 30 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of real images: 8598 |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Energyfilter slit width: 20 eV : GIF Bioquantum / Energyfilter slit width: 20 eV |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 2087449 | ||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||
3D reconstruction | Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 1032305 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||
| Atomic model building | Details: used dB:STP atomic model / Source name: Other / Type: experimental model | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj









































