+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8swf | ||||||
|---|---|---|---|---|---|---|---|
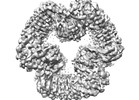
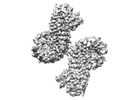
| Title | Cryo-EM structure of NLRP3 open octamer | ||||||
 Components Components | NACHT, LRR and PYD domains-containing protein 3 | ||||||
 Keywords Keywords |  IMMUNE SYSTEM / Activated NLRP3 / IMMUNE SYSTEM / Activated NLRP3 /  Cryo-EM Cryo-EM | ||||||
| Function / homology |  Function and homology information Function and homology informationsmall molecule sensor activity / detection of biotic stimulus / phosphatidylinositol phosphate binding / cysteine-type endopeptidase activator activity / positive regulation of T-helper 2 cell differentiation / interphase microtubule organizing center / NLRP3 inflammasome complex assembly / positive regulation of T-helper 2 cell cytokine production /  NLRP3 inflammasome complex / positive regulation of type 2 immune response ...small molecule sensor activity / detection of biotic stimulus / phosphatidylinositol phosphate binding / cysteine-type endopeptidase activator activity / positive regulation of T-helper 2 cell differentiation / interphase microtubule organizing center / NLRP3 inflammasome complex assembly / positive regulation of T-helper 2 cell cytokine production / NLRP3 inflammasome complex / positive regulation of type 2 immune response ...small molecule sensor activity / detection of biotic stimulus / phosphatidylinositol phosphate binding / cysteine-type endopeptidase activator activity / positive regulation of T-helper 2 cell differentiation / interphase microtubule organizing center / NLRP3 inflammasome complex assembly / positive regulation of T-helper 2 cell cytokine production /  NLRP3 inflammasome complex / positive regulation of type 2 immune response / osmosensory signaling pathway / NLRP3 inflammasome complex / positive regulation of type 2 immune response / osmosensory signaling pathway /  peptidoglycan binding / phosphatidylinositol-4-phosphate binding / negative regulation of non-canonical NF-kappaB signal transduction / peptidoglycan binding / phosphatidylinositol-4-phosphate binding / negative regulation of non-canonical NF-kappaB signal transduction /  microtubule organizing center / negative regulation of interleukin-1 beta production / pattern recognition receptor signaling pathway / negative regulation of NF-kappaB transcription factor activity / microtubule organizing center / negative regulation of interleukin-1 beta production / pattern recognition receptor signaling pathway / negative regulation of NF-kappaB transcription factor activity /  pyroptosis / positive regulation of cysteine-type endopeptidase activity involved in apoptotic process / protein maturation / The NLRP3 inflammasome / negative regulation of acute inflammatory response / positive regulation of interleukin-4 production / signaling adaptor activity / pyroptosis / positive regulation of cysteine-type endopeptidase activity involved in apoptotic process / protein maturation / The NLRP3 inflammasome / negative regulation of acute inflammatory response / positive regulation of interleukin-4 production / signaling adaptor activity /  Purinergic signaling in leishmaniasis infection / molecular condensate scaffold activity / positive regulation of interleukin-1 beta production / Purinergic signaling in leishmaniasis infection / molecular condensate scaffold activity / positive regulation of interleukin-1 beta production /  ADP binding / ADP binding /  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / protein homooligomerization / defense response / Cytoprotection by HMOX1 / Metalloprotease DUBs / negative regulation of inflammatory response / cellular response to virus / positive regulation of inflammatory response / positive regulation of non-canonical NF-kappaB signal transduction / SARS-CoV-1 activates/modulates innate immune responses / positive regulation of NF-kappaB transcription factor activity / DNA-binding transcription factor binding / cellular response to lipopolysaccharide / sequence-specific DNA binding / molecular adaptor activity / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / protein homooligomerization / defense response / Cytoprotection by HMOX1 / Metalloprotease DUBs / negative regulation of inflammatory response / cellular response to virus / positive regulation of inflammatory response / positive regulation of non-canonical NF-kappaB signal transduction / SARS-CoV-1 activates/modulates innate immune responses / positive regulation of NF-kappaB transcription factor activity / DNA-binding transcription factor binding / cellular response to lipopolysaccharide / sequence-specific DNA binding / molecular adaptor activity /  inflammatory response / inflammatory response /  Golgi membrane / Golgi membrane /  innate immune response / apoptotic process / SARS-CoV-2 activates/modulates innate and adaptive immune responses / innate immune response / apoptotic process / SARS-CoV-2 activates/modulates innate and adaptive immune responses /  endoplasmic reticulum / endoplasmic reticulum /  signal transduction / signal transduction /  ATP hydrolysis activity / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / positive regulation of transcription by RNA polymerase II /  mitochondrion / extracellular region / mitochondrion / extracellular region /  ATP binding / ATP binding /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.39 Å cryo EM / Resolution: 3.39 Å | ||||||
 Authors Authors | Yu, X. / Matico, R.E. / Miller, R. / Schoubroeck, B.V. / Grauwen, K. / Suarez, J. / Pietrak, B. / Haloi, N. / Yin, Y. / Tresadern, G.J. ...Yu, X. / Matico, R.E. / Miller, R. / Schoubroeck, B.V. / Grauwen, K. / Suarez, J. / Pietrak, B. / Haloi, N. / Yin, Y. / Tresadern, G.J. / Perez-Benito, L. / Lindahl, E. / Bottelbergs, A. / Oehlrich, D. / Opdenbosch, N.V. / Sharma, S. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Cryo-EM structures of NLRP3 reveal its self-activation mechanism Authors: Yu, X. / Matico, R.E. / Miller, R. / Schoubroeck, B.V. / Grauwen, K. / Suarez, J. / Pietrak, B. / Haloi, N. / Yin, Y. / Tresadern, G.J. / Perez-Benito, L. / Lindahl, E. / Bottelbergs, A. / ...Authors: Yu, X. / Matico, R.E. / Miller, R. / Schoubroeck, B.V. / Grauwen, K. / Suarez, J. / Pietrak, B. / Haloi, N. / Yin, Y. / Tresadern, G.J. / Perez-Benito, L. / Lindahl, E. / Bottelbergs, A. / Oehlrich, D. / Opdenbosch, N.V. / Sharma, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8swf.cif.gz 8swf.cif.gz | 1.2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8swf.ent.gz pdb8swf.ent.gz | 1 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8swf.json.gz 8swf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sw/8swf https://data.pdbj.org/pub/pdb/validation_reports/sw/8swf ftp://data.pdbj.org/pub/pdb/validation_reports/sw/8swf ftp://data.pdbj.org/pub/pdb/validation_reports/sw/8swf | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  40811MC  8swkC  8sxnC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 103675.742 Da / Num. of mol.: 8 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: NLRP3, C1orf7, CIAS1, NALP3, PYPAF1 / Production host: Homo sapiens (human) / Gene: NLRP3, C1orf7, CIAS1, NALP3, PYPAF1 / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm)References: UniProt: Q96P20,  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: NLRP3 open octamer complex / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2400 nm / Nominal defocus min: 1200 nm Bright-field microscopy / Nominal defocus max: 2400 nm / Nominal defocus min: 1200 nm |
| Image recording | Electron dose: 52 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
3D reconstruction | Resolution: 3.39 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 466664 / Symmetry type: POINT |
 Movie
Movie Controller
Controller



 PDBj
PDBj













