[English] 日本語
 Yorodumi
Yorodumi- PDB-8k9f: Cryo-EM structure of the photosynthetic alternative complex III f... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8k9f | ||||||
|---|---|---|---|---|---|---|---|
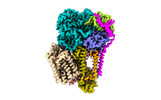
| Title | Cryo-EM structure of the photosynthetic alternative complex III from Chloroflexus aurantiacus at 2.9 angstrom | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / Photosynthetic alternative complex III MEMBRANE PROTEIN / Photosynthetic alternative complex III | ||||||
| Function / homology |  Function and homology information Function and homology information electron transfer activity / electron transfer activity /  heme binding / heme binding /  membrane / membrane /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |    Chloroflexus aurantiacus (bacteria) Chloroflexus aurantiacus (bacteria) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.9 Å cryo EM / Resolution: 2.9 Å | ||||||
 Authors Authors | Xu, X. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: Plant Cell / Year: 2024 Journal: Plant Cell / Year: 2024Title: Cryo-EM structure of HQNO-bound Alternative Complex III from the anoxygenic phototrophic bacterium Chloroflexus aurantiacus. Authors: Jiyu Xin / Zhenzhen Min / Lu Yu / Xinyi Yuan / Aokun Liu / Wenping Wu / Xin Zhang / Huimin He / Jingyi Wu / Yueyong Xin / Robert E Blankenship / Changlin Tian / Xiaoling Xu /   Abstract: Alternative complex III (ACIII) couples quinol oxidation and electron acceptor reduction with potential transmembrane proton translocation. It is compositionally and structurally different from the ...Alternative complex III (ACIII) couples quinol oxidation and electron acceptor reduction with potential transmembrane proton translocation. It is compositionally and structurally different from the cytochrome bc1/b6f complexes, but functionally replaces these enzymes in the photosynthetic and/or respiratory electron transport chains (ETCs) of many bacteria. However, the true compositions and architectures of ACIIIs remain unclear, as do their structural and functional relevance in mediating the ETCs. We here determined cryogenic electron microscopy structures of photosynthetic ACIII isolated from Chloroflexus aurantiacus (CaACIIIp), in apo-form and in complexed form bound to a menadiol analog 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO). Besides six canonical subunits (ActABCDEF), the structures revealed conformations of two previously unresolved subunits, ActG and I, which contributed to the complex stability. We also elucidated the structural basis of menaquinol oxidation and subsequent electron transfer along the [3Fe-4S]-6 hemes wire to its periplasmic electron acceptors, using electron paramagnetic resonance (EPR), spectroelectrochemistry, enzymatic analyses and molecular dynamics (MD) simulations. A unique insertion loop in ActE was shown to function in determining the binding specificity of CaACIIIp for downstream electron acceptors. This study broadens our understanding of the structural diversity and molecular evolution of ACIIIs, enabling further investigation of the (mena)quinol oxidoreductases evolved coupling mechanism in bacterial energy conservation. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8k9f.cif.gz 8k9f.cif.gz | 513.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8k9f.ent.gz pdb8k9f.ent.gz | 405 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8k9f.json.gz 8k9f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k9/8k9f https://data.pdbj.org/pub/pdb/validation_reports/k9/8k9f ftp://data.pdbj.org/pub/pdb/validation_reports/k9/8k9f ftp://data.pdbj.org/pub/pdb/validation_reports/k9/8k9f | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36985MC  8k9eC  8x2jC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 5 types, 5 molecules ABCEG
| #1: Protein | Mass: 25247.039 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV2 |
|---|---|
| #2: Protein | Mass: 113115.359 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV3 |
| #3: Protein | Mass: 55223.520 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV4 |
| #5: Protein | Mass: 23017.016 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV6 |
| #7: Protein |  Mass: 12485.551 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV8 |
-Quinol:cytochrome c oxidoreductase ... , 2 types, 2 molecules DF
| #4: Protein | Mass: 19648.570 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV5 |
|---|---|
| #6: Protein | Mass: 45721.672 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria)References: UniProt: A9WEV7 |
-Protein/peptide , 1 types, 1 molecules I
| #8: Protein/peptide | Mass: 4322.134 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) |
|---|
-Non-polymers , 7 types, 14 molecules 






| #9: Chemical | ChemComp-HEC /  Heme C Heme C#10: Chemical |  Iron–sulfur cluster Iron–sulfur cluster#11: Chemical | ChemComp-F3S / |  Iron–sulfur cluster Iron–sulfur cluster#12: Chemical | ChemComp-MG / | #13: Chemical | ChemComp-JLQ / [( | Mass: 663.906 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C35H70NO8P #14: Chemical | ChemComp-JL3 / [( | Mass: 663.906 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C35H70NO8P #15: Chemical | ChemComp-JM9 / | Mass: 765.240 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C48H92O6 |
|---|
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Alternative complex III / Type: COMPLEX / Entity ID: #1-#8 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1800 nm / Nominal defocus min: 1000 nm Bright-field microscopy / Nominal defocus max: 1800 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 2.9 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 103633 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj





















