[English] 日本語
 Yorodumi
Yorodumi- EMDB-36985: Cryo-EM structure of the photosynthetic alternative complex III f... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the photosynthetic alternative complex III from Chloroflexus aurantiacus at 2.9 angstrom | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Photosynthetic alternative complex III /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information electron transfer activity / electron transfer activity /  heme binding / heme binding /  membrane / membrane /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |    Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) Chloroflexus aurantiacus (strain ATCC 29366 / DSM 635 / J-10-fl) (bacteria) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.9 Å cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Xu X | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Plant Cell / Year: 2024 Journal: Plant Cell / Year: 2024Title: Cryo-EM structure of HQNO-bound Alternative Complex III from the anoxygenic phototrophic bacterium Chloroflexus aurantiacus. Authors: Jiyu Xin / Zhenzhen Min / Lu Yu / Xinyi Yuan / Aokun Liu / Wenping Wu / Xin Zhang / Huimin He / Jingyi Wu / Yueyong Xin / Robert E Blankenship / Changlin Tian / Xiaoling Xu /   Abstract: Alternative complex III (ACIII) couples quinol oxidation and electron acceptor reduction with potential transmembrane proton translocation. It is compositionally and structurally different from the ...Alternative complex III (ACIII) couples quinol oxidation and electron acceptor reduction with potential transmembrane proton translocation. It is compositionally and structurally different from the cytochrome bc1/b6f complexes, but functionally replaces these enzymes in the photosynthetic and/or respiratory electron transport chains (ETCs) of many bacteria. However, the true compositions and architectures of ACIIIs remain unclear, as do their structural and functional relevance in mediating the ETCs. We here determined cryogenic electron microscopy structures of photosynthetic ACIII isolated from Chloroflexus aurantiacus (CaACIIIp), in apo-form and in complexed form bound to a menadiol analog 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO). Besides six canonical subunits (ActABCDEF), the structures revealed conformations of two previously unresolved subunits, ActG and I, which contributed to the complex stability. We also elucidated the structural basis of menaquinol oxidation and subsequent electron transfer along the [3Fe-4S]-6 hemes wire to its periplasmic electron acceptors, using electron paramagnetic resonance (EPR), spectroelectrochemistry, enzymatic analyses and molecular dynamics (MD) simulations. A unique insertion loop in ActE was shown to function in determining the binding specificity of CaACIIIp for downstream electron acceptors. This study broadens our understanding of the structural diversity and molecular evolution of ACIIIs, enabling further investigation of the (mena)quinol oxidoreductases evolved coupling mechanism in bacterial energy conservation. | |||||||||
| History |
|
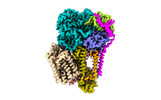
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36985.map.gz emd_36985.map.gz | 36 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36985-v30.xml emd-36985-v30.xml emd-36985.xml emd-36985.xml | 24.9 KB 24.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36985.png emd_36985.png | 190 KB | ||
| Filedesc metadata |  emd-36985.cif.gz emd-36985.cif.gz | 7.8 KB | ||
| Others |  emd_36985_half_map_1.map.gz emd_36985_half_map_1.map.gz emd_36985_half_map_2.map.gz emd_36985_half_map_2.map.gz | 29.6 MB 29.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36985 http://ftp.pdbj.org/pub/emdb/structures/EMD-36985 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36985 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36985 | HTTPS FTP |
-Related structure data
| Related structure data |  8k9fMC  8k9eC  8x2jC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36985.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36985.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36985_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
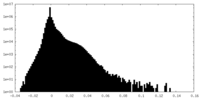
| Density Histograms |
-Half map: #1
| File | emd_36985_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Alternative complex III
+Supramolecule #1: Alternative complex III
+Macromolecule #1: Cytochrome c7-like domain-containing protein
+Macromolecule #2: Fe-S-cluster-containing hydrogenase components 1-like protein
+Macromolecule #3: Polysulphide reductase NrfD
+Macromolecule #4: Quinol:cytochrome c oxidoreductase membrane protein
+Macromolecule #5: Cytochrome c domain-containing protein
+Macromolecule #6: Quinol:cytochrome c oxidoreductase quinone-binding subunit 2
+Macromolecule #7: hypothetical protein
+Macromolecule #8: unknown
+Macromolecule #9: HEME C
+Macromolecule #10: IRON/SULFUR CLUSTER
+Macromolecule #11: FE3-S4 CLUSTER
+Macromolecule #12: MAGNESIUM ION
+Macromolecule #13: [(2~{R})-3-[2-azanylethoxy(oxidanyl)phosphoryl]oxy-2-tetradecanoy...
+Macromolecule #14: [(2~{R})-3-[2-azanylethoxy(oxidanyl)phosphoryl]oxy-2-pentadecanoy...
+Macromolecule #15: 1,3-bis(13-methyltetradecanoyloxy)propan-2-yl pentadecanoate
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.9 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 103633 |
 Movie
Movie Controller
Controller





















 Z
Z Y
Y X
X