[English] 日本語
 Yorodumi
Yorodumi- PDB-8fh3: Human IFT-A complex structures provide molecular insights into ci... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8fh3 | ||||||
|---|---|---|---|---|---|---|---|
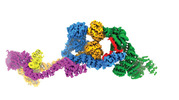
| Title | Human IFT-A complex structures provide molecular insights into ciliary transport | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TRANSPORT PROTEIN / IFT-A complex / TULP3 / TRANSPORT PROTEIN / IFT-A complex / TULP3 /  cilia cilia | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of smoothened signaling pathway involved in ventral spinal cord patterning / intraciliary transport particle A binding / smoothened signaling pathway involved in dorsal/ventral neural tube patterning / myotome development / negative regulation of smoothened signaling pathway involved in dorsal/ventral neural tube patterning / : / ear morphogenesis / intraciliary transport particle A / intraciliary anterograde transport / ganglion development ...negative regulation of smoothened signaling pathway involved in ventral spinal cord patterning / intraciliary transport particle A binding / smoothened signaling pathway involved in dorsal/ventral neural tube patterning / myotome development / negative regulation of smoothened signaling pathway involved in dorsal/ventral neural tube patterning / : / ear morphogenesis / intraciliary transport particle A / intraciliary anterograde transport / ganglion development / cone photoreceptor outer segment / bronchus morphogenesis /  digestive system development / embryonic heart tube left/right pattern formation / embryonic body morphogenesis / embryonic neurocranium morphogenesis / photoreceptor cell outer segment organization / 9+0 non-motile cilium / digestive system development / embryonic heart tube left/right pattern formation / embryonic body morphogenesis / embryonic neurocranium morphogenesis / photoreceptor cell outer segment organization / 9+0 non-motile cilium /  central nervous system neuron differentiation / neural tube patterning / intraciliary retrograde transport / protein localization to ciliary membrane / embryonic camera-type eye development / intraciliary transport / establishment of protein localization to organelle / gonad development / central nervous system neuron differentiation / neural tube patterning / intraciliary retrograde transport / protein localization to ciliary membrane / embryonic camera-type eye development / intraciliary transport / establishment of protein localization to organelle / gonad development /  regulation of cilium assembly / spinal cord dorsal/ventral patterning / photoreceptor connecting cilium / ciliary tip / regulation of cilium assembly / spinal cord dorsal/ventral patterning / photoreceptor connecting cilium / ciliary tip /  Intraflagellar transport / camera-type eye morphogenesis / embryonic brain development / regulation of G protein-coupled receptor signaling pathway / protein localization to cilium / non-motile cilium assembly / non-motile cilium / embryonic heart tube development / regulation of smoothened signaling pathway / embryonic cranial skeleton morphogenesis / Intraflagellar transport / camera-type eye morphogenesis / embryonic brain development / regulation of G protein-coupled receptor signaling pathway / protein localization to cilium / non-motile cilium assembly / non-motile cilium / embryonic heart tube development / regulation of smoothened signaling pathway / embryonic cranial skeleton morphogenesis /  nervous system process / nervous system process /  motile cilium / embryonic forelimb morphogenesis / determination of left/right symmetry / embryonic limb morphogenesis / motile cilium / embryonic forelimb morphogenesis / determination of left/right symmetry / embryonic limb morphogenesis /  limb development / anterior/posterior pattern specification / ciliary base / embryonic digit morphogenesis / receptor clustering / limb development / anterior/posterior pattern specification / ciliary base / embryonic digit morphogenesis / receptor clustering /  axoneme / axoneme /  cilium assembly / photoreceptor outer segment / Hedgehog 'off' state / negative regulation of smoothened signaling pathway / cilium assembly / photoreceptor outer segment / Hedgehog 'off' state / negative regulation of smoothened signaling pathway /  phosphatidylinositol-4,5-bisphosphate binding / phosphatidylinositol-4,5-bisphosphate binding /  centriole / cellular response to leukemia inhibitory factor / centriole / cellular response to leukemia inhibitory factor /  phosphatidylinositol binding / ciliary basal body / G protein-coupled receptor binding / neural tube closure / phosphatidylinositol binding / ciliary basal body / G protein-coupled receptor binding / neural tube closure /  brain development / brain development /  bone development / cell morphogenesis / bone development / cell morphogenesis /  cilium / cilium /  heart development / protein-containing complex assembly / in utero embryonic development / heart development / protein-containing complex assembly / in utero embryonic development /  cytoskeleton / G protein-coupled receptor signaling pathway / cytoskeleton / G protein-coupled receptor signaling pathway /  centrosome / protein-containing complex binding / centrosome / protein-containing complex binding /  nucleolus / regulation of DNA-templated transcription / nucleolus / regulation of DNA-templated transcription /  enzyme binding / extracellular region / enzyme binding / extracellular region /  nucleoplasm / nucleoplasm /  membrane / membrane /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.3 Å cryo EM / Resolution: 4.3 Å | ||||||
 Authors Authors | Jiang, M. / Palicharla, V.R. / Miller, D. / Hwang, S.H. / Zhu, H. / Hixson, P. / Mukhopadhyay, S. / Sun, J. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Cell Res / Year: 2023 Journal: Cell Res / Year: 2023Title: Human IFT-A complex structures provide molecular insights into ciliary transport. Authors: Meiqin Jiang / Vivek Reddy Palicharla / Darcie Miller / Sun-Hee Hwang / Hanwen Zhu / Patricia Hixson / Saikat Mukhopadhyay / Ji Sun /  Abstract: Intraflagellar transport (IFT) complexes, IFT-A and IFT-B, form bidirectional trains that move along the axonemal microtubules and are essential for assembling and maintaining cilia. Mutations in IFT ...Intraflagellar transport (IFT) complexes, IFT-A and IFT-B, form bidirectional trains that move along the axonemal microtubules and are essential for assembling and maintaining cilia. Mutations in IFT subunits lead to numerous ciliopathies involving multiple tissues. However, how IFT complexes assemble and mediate cargo transport lacks mechanistic understanding due to missing high-resolution structural information of the holo-complexes. Here we report cryo-EM structures of human IFT-A complexes in the presence and absence of TULP3 at overall resolutions of 3.0-3.9 Å. IFT-A adopts a "lariat" shape with interconnected core and peripheral subunits linked by structurally vital zinc-binding domains. TULP3, the cargo adapter, interacts with IFT-A through its N-terminal region, and interface mutations disrupt cargo transport. We also determine the molecular impacts of disease mutations on complex formation and ciliary transport. Our work reveals IFT-A architecture, sheds light on ciliary transport and IFT train formation, and enables the rationalization of disease mutations in ciliopathies. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8fh3.cif.gz 8fh3.cif.gz | 658.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8fh3.ent.gz pdb8fh3.ent.gz | 499.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8fh3.json.gz 8fh3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fh/8fh3 https://data.pdbj.org/pub/pdb/validation_reports/fh/8fh3 ftp://data.pdbj.org/pub/pdb/validation_reports/fh/8fh3 ftp://data.pdbj.org/pub/pdb/validation_reports/fh/8fh3 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 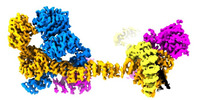 29078MC  8fgwC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-WD repeat-containing protein ... , 2 types, 2 molecules AC
| #1: Protein | Mass: 133705.641 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: WDR35, IFT121, KIAA1336 / Production host: Homo sapiens (human) / Gene: WDR35, IFT121, KIAA1336 / Production host:   Homo sapiens (human) / References: UniProt: Q9P2L0 Homo sapiens (human) / References: UniProt: Q9P2L0 |
|---|---|
| #3: Protein | Mass: 151760.391 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: WDR19, IFT144, KIAA1638 / Production host: Homo sapiens (human) / Gene: WDR19, IFT144, KIAA1638 / Production host:   Homo sapiens (human) / References: UniProt: Q8NEZ3 Homo sapiens (human) / References: UniProt: Q8NEZ3 |
-Intraflagellar transport protein ... , 2 types, 2 molecules BE
| #2: Protein |  / WD repeat-containing protein 10 / WD repeat-containing protein 140 / WD repeat-containing protein 10 / WD repeat-containing protein 140Mass: 141993.703 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: IFT122, SPG, WDR10, WDR140 / Production host: Homo sapiens (human) / Gene: IFT122, SPG, WDR10, WDR140 / Production host:   Homo sapiens (human) / References: UniProt: Q9HBG6 Homo sapiens (human) / References: UniProt: Q9HBG6 |
|---|---|
| #4: Protein |  / WD and tetratricopeptide repeats protein 2 / WD and tetratricopeptide repeats protein 2Mass: 165404.281 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: IFT140, KIAA0590, WDTC2 / Production host: Homo sapiens (human) / Gene: IFT140, KIAA0590, WDTC2 / Production host:   Homo sapiens (human) / References: UniProt: Q96RY7 Homo sapiens (human) / References: UniProt: Q96RY7 |
-Protein / Non-polymers , 2 types, 5 molecules I

| #5: Protein | Mass: 49710.059 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: TULP3, TUBL3 / Production host: Homo sapiens (human) / Gene: TULP3, TUBL3 / Production host:   Homo sapiens (human) / References: UniProt: O75386 Homo sapiens (human) / References: UniProt: O75386 |
|---|---|
| #6: Chemical | ChemComp-ZN / |
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| ||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 600 nm Bright-field microscopy / Nominal defocus max: 2000 nm / Nominal defocus min: 600 nm |
| Image recording | Electron dose: 53.06 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
CTF correction | Type: NONE |
|---|---|
3D reconstruction | Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 113784 / Symmetry type: POINT |
 Movie
Movie Controller
Controller


 PDBj
PDBj




