[English] 日本語
 Yorodumi
Yorodumi- PDB-8d51: Parathyroid hormone 1 receptor extracellular domain complexed wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8d51 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Parathyroid hormone 1 receptor extracellular domain complexed with a peptide ligand containing beta-3-homotryptophan | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  SIGNALING PROTEIN / SIGNALING PROTEIN /  parathyroid hormone 1 receptor / parathyroid hormone 1 receptor /  signaling / beta-amino acid signaling / beta-amino acid | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of chondrocyte development / regulation of chondrocyte differentiation /  parathyroid hormone receptor activity / cAMP metabolic process / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding ...negative regulation of chondrocyte development / regulation of chondrocyte differentiation / parathyroid hormone receptor activity / cAMP metabolic process / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding ...negative regulation of chondrocyte development / regulation of chondrocyte differentiation /  parathyroid hormone receptor activity / cAMP metabolic process / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding / parathyroid hormone receptor activity / cAMP metabolic process / G protein-coupled peptide receptor activity / negative regulation of chondrocyte differentiation / Class B/2 (Secretin family receptors) / osteoblast development / positive regulation of inositol phosphate biosynthetic process / peptide hormone receptor binding /  bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / bone mineralization / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger /  peptide hormone binding / epidermis development / chondrocyte differentiation / cell maturation / peptide hormone binding / epidermis development / chondrocyte differentiation / cell maturation /  bone resorption / bone resorption /  skeletal system development / female pregnancy / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / skeletal system development / female pregnancy / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway /  hormone activity / intracellular calcium ion homeostasis / : / cell-cell signaling / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (s) signalling events / hormone activity / intracellular calcium ion homeostasis / : / cell-cell signaling / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (s) signalling events /  regulation of gene expression / basolateral plasma membrane / cell population proliferation / in utero embryonic development / cell surface receptor signaling pathway / regulation of gene expression / basolateral plasma membrane / cell population proliferation / in utero embryonic development / cell surface receptor signaling pathway /  receptor complex / apical plasma membrane / G protein-coupled receptor signaling pathway / negative regulation of cell population proliferation / positive regulation of cell population proliferation / receptor complex / apical plasma membrane / G protein-coupled receptor signaling pathway / negative regulation of cell population proliferation / positive regulation of cell population proliferation /  Golgi apparatus / protein homodimerization activity / Golgi apparatus / protein homodimerization activity /  extracellular space / extracellular region / extracellular space / extracellular region /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Yu, Z. / Kreitler, D.F. / Gellman, S.H. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Angew.Chem.Int.Ed.Engl. / Year: 2023 Journal: Angew.Chem.Int.Ed.Engl. / Year: 2023Title: Harnessing Aromatic-Histidine Interactions through Synergistic Backbone Extension and Side Chain Modification. Authors: Yu, Z. / Kreitler, D.F. / Chiu, Y.T.T. / Xu, R. / Bruchs, A.T. / Bingman, C.A. / Gellman, S.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8d51.cif.gz 8d51.cif.gz | 79.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8d51.ent.gz pdb8d51.ent.gz | 49 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8d51.json.gz 8d51.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d5/8d51 https://data.pdbj.org/pub/pdb/validation_reports/d5/8d51 ftp://data.pdbj.org/pub/pdb/validation_reports/d5/8d51 ftp://data.pdbj.org/pub/pdb/validation_reports/d5/8d51 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 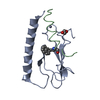 8d52C  3h3gS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 11995.670 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: PTH1R, PTHR, PTHR1 / Production host: Homo sapiens (human) / Gene: PTH1R, PTHR, PTHR1 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q03431 Escherichia coli (E. coli) / References: UniProt: Q03431 |
|---|---|
| #2: Protein/peptide | Mass: 2822.272 Da / Num. of mol.: 1 / Mutation: I31 substituted with beta-3-tryptophan / Source method: obtained synthetically / Details: synthetic peptide / Source: (synth.)   Homo sapiens (human) / References: UniProt: P12272 Homo sapiens (human) / References: UniProt: P12272 |
| #3: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol |
| #4: Chemical | ChemComp-ZN / |
| #5: Water | ChemComp-HOH /  Water Water |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.35 Å3/Da / Density % sol: 47.56 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8 Details: 28 % v/v PEG Smear Low, 0.1 M tris-HCl pH 8, 0.15 M Sodium citrate, 1% ethylene glycol, 1 mM ZnSO4 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS-II NSLS-II  / Beamline: 17-ID-1 / Wavelength: 0.920105 Å / Beamline: 17-ID-1 / Wavelength: 0.920105 Å |
| Detector | Type: DECTRIS EIGER X 9M / Detector: PIXEL / Date: Apr 6, 2022 / Details: KB |
| Radiation | Monochromator: DCM / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.920105 Å / Relative weight: 1 : 0.920105 Å / Relative weight: 1 |
| Reflection | Resolution: 1.997→49.179 Å / Num. obs: 7236 / % possible obs: 75.2 % / Redundancy: 5.4 % / Biso Wilson estimate: 53.73 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.052 / Rpim(I) all: 0.027 / Rrim(I) all: 0.063 / Net I/σ(I): 12.6 |
| Reflection shell | Resolution: 1.997→2.206 Å / Rmerge(I) obs: 0.831 / Mean I/σ(I) obs: 1.5 / Num. unique obs: 362 / CC1/2: 0.843 / Rpim(I) all: 0.628 / Rrim(I) all: 0.947 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3H3G Resolution: 2→36.14 Å / SU ML: 0.2019 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 36.5025 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 69.45 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→36.14 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller


 PDBj
PDBj





