+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7zvl | ||||||
|---|---|---|---|---|---|---|---|
| Title | HUMAN PRMT5:MEP50 Crystal Structure With MTA and Fragment Bound | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  MTAP / MTAP /  methyl transferase / methyl transferase /  fragment-based lead discovery / FBDD fragment-based lead discovery / FBDD | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of adenylate cyclase-inhibiting dopamine receptor signaling pathway / peptidyl-arginine N-methylation / oocyte axis specification / type II protein arginine methyltransferase / protein-arginine omega-N symmetric methyltransferase activity / peptidyl-arginine methylation / Golgi ribbon formation / negative regulation of epithelial cell proliferation involved in prostate gland development / histone H4R3 methyltransferase activity / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development ...positive regulation of adenylate cyclase-inhibiting dopamine receptor signaling pathway / peptidyl-arginine N-methylation / oocyte axis specification / type II protein arginine methyltransferase / protein-arginine omega-N symmetric methyltransferase activity / peptidyl-arginine methylation / Golgi ribbon formation / negative regulation of epithelial cell proliferation involved in prostate gland development / histone H4R3 methyltransferase activity / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development / epithelial cell proliferation involved in prostate gland development / histone arginine N-methyltransferase activity / methylosome / protein-arginine N-methyltransferase activity / methyl-CpG binding / positive regulation of mRNA splicing, via spliceosome / : /  endothelial cell activation / histone H3 methyltransferase activity / Cul4B-RING E3 ubiquitin ligase complex / regulation of mitotic nuclear division / endothelial cell activation / histone H3 methyltransferase activity / Cul4B-RING E3 ubiquitin ligase complex / regulation of mitotic nuclear division /  histone methyltransferase complex / positive regulation of oligodendrocyte differentiation / histone methyltransferase complex / positive regulation of oligodendrocyte differentiation /  histone methyltransferase activity / histone methyltransferase activity /  E-box binding / negative regulation of cell differentiation / ubiquitin-like ligase-substrate adaptor activity / spliceosomal snRNP assembly / ribonucleoprotein complex binding / regulation of ERK1 and ERK2 cascade / nuclear receptor coactivator activity / regulation of signal transduction by p53 class mediator / E-box binding / negative regulation of cell differentiation / ubiquitin-like ligase-substrate adaptor activity / spliceosomal snRNP assembly / ribonucleoprotein complex binding / regulation of ERK1 and ERK2 cascade / nuclear receptor coactivator activity / regulation of signal transduction by p53 class mediator /  methyltransferase activity / methyltransferase activity /  liver regeneration / DNA-templated transcription termination / circadian regulation of gene expression / Regulation of TP53 Activity through Methylation / RMTs methylate histone arginines / protein polyubiquitination / transcription corepressor activity / liver regeneration / DNA-templated transcription termination / circadian regulation of gene expression / Regulation of TP53 Activity through Methylation / RMTs methylate histone arginines / protein polyubiquitination / transcription corepressor activity /  p53 binding / p53 binding /  snRNP Assembly / ubiquitin-dependent protein catabolic process / snRNP Assembly / ubiquitin-dependent protein catabolic process /  chromatin remodeling / protein heterodimerization activity / positive regulation of cell population proliferation / chromatin remodeling / protein heterodimerization activity / positive regulation of cell population proliferation /  chromatin / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / chromatin / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II /  Golgi apparatus / Golgi apparatus /  nucleoplasm / identical protein binding / nucleoplasm / identical protein binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.39 Å MOLECULAR REPLACEMENT / Resolution: 2.39 Å | ||||||
 Authors Authors | Ahmad, M.U. / Koelmel, W. / Arkhipova, V. / Lawson, J.D. / Smith, C.R. / Gunn, R.J. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Rsc Med Chem / Year: 2022 Journal: Rsc Med Chem / Year: 2022Title: Fragment optimization and elaboration strategies - the discovery of two lead series of PRMT5/MTA inhibitors from five fragment hits. Authors: Smith, C.R. / Kulyk, S. / Ahmad, M.U.D. / Arkhipova, V. / Christensen, J.G. / Gunn, R.J. / Ivetac, A. / Ketcham, J.M. / Kuehler, J. / Lawson, J.D. / Thomas, N.C. / Wang, X. / Marx, M.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7zvl.cif.gz 7zvl.cif.gz | 392.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7zvl.ent.gz pdb7zvl.ent.gz | 318.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7zvl.json.gz 7zvl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zv/7zvl https://data.pdbj.org/pub/pdb/validation_reports/zv/7zvl ftp://data.pdbj.org/pub/pdb/validation_reports/zv/7zvl ftp://data.pdbj.org/pub/pdb/validation_reports/zv/7zvl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7uy1C  7uyfC  7zupC  7zuqC  7zuuC  7zuyC  7zv2C  7zvuC  8csgC  8ctbC  5emlS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 73763.625 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host: Homo sapiens (human) / Gene: PRMT5, HRMT1L5, IBP72, JBP1, SKB1 / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm)References: UniProt: O14744, type II protein arginine methyltransferase |
|---|---|
| #2: Protein |  WD repeat-containing protein 77 / MEP-50 / Androgen receptor cofactor p44 / WD repeat-containing protein 77 / p44/Mep50 WD repeat-containing protein 77 / MEP-50 / Androgen receptor cofactor p44 / WD repeat-containing protein 77 / p44/Mep50Mass: 37862.406 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host: Homo sapiens (human) / Gene: WDR77, MEP50, WD45, HKMT1069, Nbla10071 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: Q9BQA1 Spodoptera frugiperda (fall armyworm) / References: UniProt: Q9BQA1 |
-Non-polymers , 5 types, 140 molecules 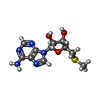






| #3: Chemical | ChemComp-MTA /  5′-Methylthioadenosine 5′-Methylthioadenosine | ||||
|---|---|---|---|---|---|
| #4: Chemical | ChemComp-UNL / Num. of mol.: 1 / Source method: obtained synthetically / Feature type: SUBJECT OF INVESTIGATION | ||||
| #5: Chemical |  Glycerol Glycerol#6: Chemical | ChemComp-CL / |  Chloride Chloride#7: Water | ChemComp-HOH / |  Water Water |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.78 Å3/Da / Density % sol: 55.79 % |
|---|---|
Crystal grow | Temperature: 281 K / Method: vapor diffusion, sitting drop Details: 16% PEG3350, 100 mM Na citrate pH 5.4, 100mM Carboxylic acids |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9197 Å / Beamline: I03 / Wavelength: 0.9197 Å |
| Detector | Type: DECTRIS EIGER2 XE 16M / Detector: PIXEL / Date: Jul 3, 2021 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9197 Å / Relative weight: 1 : 0.9197 Å / Relative weight: 1 |
| Reflection | Resolution: 2.38→109.37 Å / Num. obs: 21837 / % possible obs: 85.56 % / Observed criterion σ(I): 1.85 / Redundancy: 11.5 % / CC1/2: 0.99 / Net I/σ(I): 12.8 |
| Reflection shell | Resolution: 2.38→2.75 Å / Num. unique obs: 1092 / CC1/2: 0.83 / % possible all: 63 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5EML Resolution: 2.39→109.37 Å / Cor.coef. Fo:Fc: 0.918 / Cor.coef. Fo:Fc free: 0.868 / SU B: 29.439 / SU ML: 0.326 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.554 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: U VALUES : WITH TLS ADDED HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 229.22 Å2 / Biso mean: 65.392 Å2 / Biso min: 7.03 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.39→109.37 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.39→2.448 Å / Rfactor Rfree error: 0
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj





