[English] 日本語
 Yorodumi
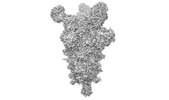
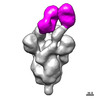
Yorodumi- PDB-7upx: Three RBD-down state of SARS-CoV-2 D614G spike in complex with th... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7upx | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Three RBD-down state of SARS-CoV-2 D614G spike in complex with the SP1-77 neutralizing antibody Fab fragment (local refinement of the RBD and Fab variable domains) | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords |  VIRAL PROTEIN VIRAL PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane ...Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / entry receptor-mediated virion attachment to host cell / receptor-mediated endocytosis of virus by host cell / Attachment and Entry /  membrane fusion / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / membrane fusion / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell /  receptor ligand activity / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / receptor ligand activity / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / viral envelope / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||||||||
| Biological species |   Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirus  Homo sapiens (human) Homo sapiens (human)  Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||||||||
 Authors Authors | Zhang, J. / Luo, S. / Kreutzberger, A. / Kirchhausen, T. / Chen, B. / Haynes, B. / Alt, F. | |||||||||||||||
| Funding support |  United States, 4items United States, 4items
| |||||||||||||||
 Citation Citation |  Journal: Sci Immunol / Year: 2022 Journal: Sci Immunol / Year: 2022Title: An antibody from single human V-rearranging mouse neutralizes all SARS-CoV-2 variants through BA.5 by inhibiting membrane fusion. Authors: Sai Luo / Jun Zhang / Alex J B Kreutzberger / Amanda Eaton / Robert J Edwards / Changbin Jing / Hai-Qiang Dai / Gregory D Sempowski / Kenneth Cronin / Robert Parks / Adam Yongxin Ye / ...Authors: Sai Luo / Jun Zhang / Alex J B Kreutzberger / Amanda Eaton / Robert J Edwards / Changbin Jing / Hai-Qiang Dai / Gregory D Sempowski / Kenneth Cronin / Robert Parks / Adam Yongxin Ye / Katayoun Mansouri / Maggie Barr / Novalia Pishesha / Aimee Chapdelaine Williams / Lucas Vieira Francisco / Anand Saminathan / Hanqin Peng / Himanshu Batra / Lorenza Bellusci / Surender Khurana / S Munir Alam / David C Montefiori / Kevin O Saunders / Ming Tian / Hidde Ploegh / Tom Kirchhausen / Bing Chen / Barton F Haynes / Frederick W Alt /  Abstract: SARS-CoV-2 Omicron subvariants have generated a worldwide health crisis due to resistance to most approved SARS-CoV-2 neutralizing antibodies and evasion of vaccination-induced antibodies. To manage ...SARS-CoV-2 Omicron subvariants have generated a worldwide health crisis due to resistance to most approved SARS-CoV-2 neutralizing antibodies and evasion of vaccination-induced antibodies. To manage Omicron subvariants and prepare for new ones, additional means of isolating broad and potent humanized SARS-CoV-2 neutralizing antibodies are desirable. Here, we describe a mouse model in which the primary B cell receptor (BCR) repertoire is generated solely through V(D)J recombination of a human V1-2 heavy chain (HC) and, substantially, a human Vκ1-33 light chain (LC). Thus, primary humanized BCR repertoire diversity in these mice derives from immensely diverse HC and LC antigen-contact CDR3 sequences generated by nontemplated junctional modifications during V(D)J recombination. Immunizing this mouse model with SARS-CoV-2 (Wuhan-Hu-1) spike protein immunogens elicited several V1-2/Vκ1-33-based neutralizing antibodies that bound RBD in a different mode from each other and from those of many prior patient-derived V1-2-based neutralizing antibodies. Of these, SP1-77 potently and broadly neutralized all SARS-CoV-2 variants through BA.5. Cryo-EM studies revealed that SP1-77 bound RBD away from the receptor-binding motif via a CDR3-dominated recognition mode. Lattice light-sheet microscopy-based studies showed that SP1-77 did not block ACE2-mediated viral attachment or endocytosis but rather blocked viral-host membrane fusion. The broad and potent SP1-77 neutralization activity and nontraditional mechanism of action suggest that it might have therapeutic potential. Likewise, the SP1-77 binding epitope may inform vaccine strategies. Last, the type of humanized mouse models that we have described may contribute to identifying therapeutic antibodies against future SARS-CoV-2 variants and other pathogens. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7upx.cif.gz 7upx.cif.gz | 168.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7upx.ent.gz pdb7upx.ent.gz | 112.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7upx.json.gz 7upx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/up/7upx https://data.pdbj.org/pub/pdb/validation_reports/up/7upx ftp://data.pdbj.org/pub/pdb/validation_reports/up/7upx ftp://data.pdbj.org/pub/pdb/validation_reports/up/7upx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  26677MC  7upwC  7upyC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  Spike protein / S glycoprotein / E2 / Peplomer protein Spike protein / S glycoprotein / E2 / Peplomer proteinMass: 144858.031 Da / Num. of mol.: 1 / Mutation: D614G Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirusGene: S, 2 / Production host:   Homo sapiens (human) / References: UniProt: P0DTC2 Homo sapiens (human) / References: UniProt: P0DTC2 |
|---|---|
| #2: Antibody | Mass: 49665.703 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)   Mus musculus (house mouse) Mus musculus (house mouse)Production host:   Homo sapiens (human) Homo sapiens (human) |
| #3: Antibody | Mass: 23294.617 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)   Mus musculus (house mouse) Mus musculus (house mouse)Production host:   Homo sapiens (human) Homo sapiens (human) |
| #4: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 570.542 Da / Num. of mol.: 1 / Mass: 570.542 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 6.5 MDa / Experimental value: NO | ||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES | ||||||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2200 nm / Nominal defocus min: 500 nm Bright-field microscopy / Nominal defocus max: 2200 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 54.91 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20_4459: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 7657522 | ||||||||||||||||||||||||
3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 250319 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: AB INITIO MODEL | ||||||||||||||||||||||||
| Atomic model building | PDB-ID: 7KRR Pdb chain-ID: A / Pdb chain residue range: 14-1211 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





 PDBj
PDBj





