+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7nyc | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
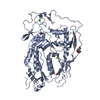
| Title | cryoEM structure of 3C9-sMAC | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords |  IMMUNE SYSTEM / Complement / IMMUNE SYSTEM / Complement /  MACPF / MACPF /  Membrane Attack Complex / CDC / pore forming Membrane Attack Complex / CDC / pore forming | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationcell killing / Terminal pathway of complement /  membrane attack complex / complement binding / other organism cell membrane / Activation of C3 and C5 / negative regulation of macrophage chemotaxis / membrane attack complex / complement binding / other organism cell membrane / Activation of C3 and C5 / negative regulation of macrophage chemotaxis /  complement activation, alternative pathway / complement activation, alternative pathway /  complement activation / complement activation /  chemokine activity ...cell killing / Terminal pathway of complement / chemokine activity ...cell killing / Terminal pathway of complement /  membrane attack complex / complement binding / other organism cell membrane / Activation of C3 and C5 / negative regulation of macrophage chemotaxis / membrane attack complex / complement binding / other organism cell membrane / Activation of C3 and C5 / negative regulation of macrophage chemotaxis /  complement activation, alternative pathway / complement activation, alternative pathway /  complement activation / complement activation /  chemokine activity / chemokine activity /  retinol binding / retinol binding /  endopeptidase inhibitor activity / positive regulation of vascular endothelial growth factor production / endopeptidase inhibitor activity / positive regulation of vascular endothelial growth factor production /  complement activation, classical pathway / positive regulation of chemokine production / Peptide ligand-binding receptors / complement activation, classical pathway / positive regulation of chemokine production / Peptide ligand-binding receptors /  Regulation of Complement cascade / protein homooligomerization / Regulation of Complement cascade / protein homooligomerization /  extracellular vesicle / extracellular vesicle /  chemotaxis / positive regulation of immune response / G alpha (i) signalling events / blood microparticle / killing of cells of another organism / in utero embryonic development / cell surface receptor signaling pathway / chemotaxis / positive regulation of immune response / G alpha (i) signalling events / blood microparticle / killing of cells of another organism / in utero embryonic development / cell surface receptor signaling pathway /  immune response / immune response /  inflammatory response / G protein-coupled receptor signaling pathway / inflammatory response / G protein-coupled receptor signaling pathway /  signaling receptor binding / signaling receptor binding /  innate immune response / protein-containing complex binding / innate immune response / protein-containing complex binding /  extracellular space / extracellular exosome / extracellular region / extracellular space / extracellular exosome / extracellular region /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.54 Å cryo EM / Resolution: 3.54 Å | ||||||||||||
 Authors Authors | Menny, A. / Couves, E.C. / Bubeck, D. | ||||||||||||
| Funding support | European Union,  United Kingdom, 3items United Kingdom, 3items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Structural basis of soluble membrane attack complex packaging for clearance. Authors: Anaïs Menny / Marie V Lukassen / Emma C Couves / Vojtech Franc / Albert J R Heck / Doryen Bubeck /   Abstract: Unregulated complement activation causes inflammatory and immunological pathologies with consequences for human disease. To prevent bystander damage during an immune response, extracellular ...Unregulated complement activation causes inflammatory and immunological pathologies with consequences for human disease. To prevent bystander damage during an immune response, extracellular chaperones (clusterin and vitronectin) capture and clear soluble precursors to the membrane attack complex (sMAC). However, how these chaperones block further polymerization of MAC and prevent the complex from binding target membranes remains unclear. Here, we address that question by combining cryo electron microscopy (cryoEM) and cross-linking mass spectrometry (XL-MS) to solve the structure of sMAC. Together our data reveal how clusterin recognizes and inhibits polymerizing complement proteins by binding a negatively charged surface of sMAC. Furthermore, we show that the pore-forming C9 protein is trapped in an intermediate conformation whereby only one of its two transmembrane β-hairpins has unfurled. This structure provides molecular details for immune pore formation and helps explain a complement control mechanism that has potential implications for how cell clearance pathways mediate immune homeostasis. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7nyc.cif.gz 7nyc.cif.gz | 1 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7nyc.ent.gz pdb7nyc.ent.gz | 843.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7nyc.json.gz 7nyc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ny/7nyc https://data.pdbj.org/pub/pdb/validation_reports/ny/7nyc ftp://data.pdbj.org/pub/pdb/validation_reports/ny/7nyc ftp://data.pdbj.org/pub/pdb/validation_reports/ny/7nyc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  12650MC  7nydC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Complement component ... , 6 types, 8 molecules CDEGHIBF
| #1: Protein | Mass: 91221.484 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P10643 Homo sapiens (human) / References: UniProt: P10643 | ||||
|---|---|---|---|---|---|
| #2: Protein | Mass: 61122.852 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P07358 Homo sapiens (human) / References: UniProt: P07358 | ||||
| #3: Protein | Mass: 61782.992 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P07357 Homo sapiens (human) / References: UniProt: P07357 | ||||
| #4: Protein | Mass: 61056.594 Da / Num. of mol.: 3 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P02748 Homo sapiens (human) / References: UniProt: P02748#5: Protein | | Mass: 102541.312 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P13671 Homo sapiens (human) / References: UniProt: P13671#7: Protein | | Mass: 20410.105 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P07360 Homo sapiens (human) / References: UniProt: P07360 |
-Protein / Non-polymers , 2 types, 4 molecules A

| #11: Chemical | | #6: Protein | |  Complement component 5 / C3 and PZP-like alpha-2-macroglobulin domain-containing protein 4 Complement component 5 / C3 and PZP-like alpha-2-macroglobulin domain-containing protein 4Mass: 186546.656 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / References: UniProt: P01031 Homo sapiens (human) / References: UniProt: P01031 |
|---|
-Sugars , 5 types, 14 molecules 



| #8: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 424.401 Da / Num. of mol.: 1 / Mass: 424.401 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source | ||||
|---|---|---|---|---|---|
| #9: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta- ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 586.542 Da / Num. of mol.: 1 / Mass: 586.542 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source | ||||
| #10: Sugar | ChemComp-BMA /  Mannose Mannose#12: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine#13: Sugar | ChemComp-FUC / |  Fucose Fucose |
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: 3C9-sMAC / Type: COMPLEX Details: This sMAC oligomer contains one copy of C5b8 and 3 copies of C9, as well as multiple copies of the chaperones vitronectin and clusterin Entity ID: #1-#7 / Source: NATURAL |
|---|---|
| Molecular weight | Value: 1 MDa / Experimental value: YES |
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Conc.: 0.065 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Instrument: FEI VITROBOT MARK III / Cryogen name: ETHANE / Humidity: 95 % / Chamber temperature: 295 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / C2 aperture diameter: 70 µm Bright-field microscopy / C2 aperture diameter: 70 µm |
| Image recording | Electron dose: 40 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.8.0253 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.54 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 85151 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | Resolution: 3.5→418.8 Å / Cor.coef. Fo:Fc: 0.891 / SU B: 16.753 / SU ML: 0.236 / ESU R: 0.166 Stereochemistry target values: MAXIMUM LIKELIHOOD WITH PHASES
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: PARAMETERS FOR MASK CACLULATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 268.658 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Total: 41391 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj