Entry Database : PDB / ID : 7jweTitle Gedatolisib bound to the PI3Kg catalytic subunit p110 gamma Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform Keywords / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 2.55 Å Authors Burke, J.E. / Rathinaswamy, M.K. / Harris, N.J. Funding support Organization Grant number Country Canadian Institutes of Health Research (CIHR)
Journal : Elife / Year : 2021Title : Disease related mutations in PI3K gamma disrupt regulatory C-terminal dynamics and reveal a path to selective inhibitors.Authors : Rathinaswamy, M.K. / Gaieb, Z. / Fleming, K.D. / Borsari, C. / Harris, N.J. / Moeller, B.J. / Wymann, M.P. / Amaro, R.E. / Burke, J.E. History Deposition Aug 25, 2020 Deposition site / Processing site Revision 1.0 Mar 17, 2021 Provider / Type Revision 1.1 Oct 18, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords TRANSFERASE / PIK3CG /
TRANSFERASE / PIK3CG /  mTOR /
mTOR /  phosphoinositide / PIP3 /
phosphoinositide / PIP3 /  PI3K / p110 /
PI3K / p110 /  SIGNALING PROTEIN
SIGNALING PROTEIN Function and homology information
Function and homology information phosphatidylinositol-4-phosphate 3-kinase / regulation of calcium ion transmembrane transport / positive regulation of acute inflammatory response / respiratory burst involved in defense response / negative regulation of cardiac muscle contraction / T cell chemotaxis ...secretory granule localization / negative regulation of triglyceride catabolic process / natural killer cell chemotaxis / neutrophil extravasation /
phosphatidylinositol-4-phosphate 3-kinase / regulation of calcium ion transmembrane transport / positive regulation of acute inflammatory response / respiratory burst involved in defense response / negative regulation of cardiac muscle contraction / T cell chemotaxis ...secretory granule localization / negative regulation of triglyceride catabolic process / natural killer cell chemotaxis / neutrophil extravasation /  phosphatidylinositol-4-phosphate 3-kinase / regulation of calcium ion transmembrane transport / positive regulation of acute inflammatory response / respiratory burst involved in defense response / negative regulation of cardiac muscle contraction / T cell chemotaxis / negative regulation of fibroblast apoptotic process / phosphatidylinositol 3-kinase complex, class IB / sphingosine-1-phosphate receptor signaling pathway / dendritic cell chemotaxis / 1-phosphatidylinositol-4-phosphate 3-kinase activity / 1-phosphatidylinositol-4,5-bisphosphate 3-kinase activity /
phosphatidylinositol-4-phosphate 3-kinase / regulation of calcium ion transmembrane transport / positive regulation of acute inflammatory response / respiratory burst involved in defense response / negative regulation of cardiac muscle contraction / T cell chemotaxis / negative regulation of fibroblast apoptotic process / phosphatidylinositol 3-kinase complex, class IB / sphingosine-1-phosphate receptor signaling pathway / dendritic cell chemotaxis / 1-phosphatidylinositol-4-phosphate 3-kinase activity / 1-phosphatidylinositol-4,5-bisphosphate 3-kinase activity /  phosphatidylinositol-4,5-bisphosphate 3-kinase / phosphatidylinositol 3-kinase complex, class IA /
phosphatidylinositol-4,5-bisphosphate 3-kinase / phosphatidylinositol 3-kinase complex, class IA /  phosphatidylinositol 3-kinase / phosphatidylinositol-3-phosphate biosynthetic process / 1-phosphatidylinositol-3-kinase activity / mast cell degranulation / Erythropoietin activates Phosphoinositide-3-kinase (PI3K) / hepatocyte apoptotic process / positive regulation of Rac protein signal transduction / regulation of cell adhesion mediated by integrin / Synthesis of PIPs at the plasma membrane / phosphatidylinositol phosphate biosynthetic process /
phosphatidylinositol 3-kinase / phosphatidylinositol-3-phosphate biosynthetic process / 1-phosphatidylinositol-3-kinase activity / mast cell degranulation / Erythropoietin activates Phosphoinositide-3-kinase (PI3K) / hepatocyte apoptotic process / positive regulation of Rac protein signal transduction / regulation of cell adhesion mediated by integrin / Synthesis of PIPs at the plasma membrane / phosphatidylinositol phosphate biosynthetic process /  regulation of angiogenesis / T cell proliferation / cellular response to cAMP / GPVI-mediated activation cascade /
regulation of angiogenesis / T cell proliferation / cellular response to cAMP / GPVI-mediated activation cascade /  ephrin receptor binding /
ephrin receptor binding /  T cell activation /
T cell activation /  neutrophil chemotaxis / positive regulation of endothelial cell migration / phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of cytokine production / positive regulation of MAP kinase activity /
neutrophil chemotaxis / positive regulation of endothelial cell migration / phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of cytokine production / positive regulation of MAP kinase activity /  platelet aggregation /
platelet aggregation /  endocytosis / G beta:gamma signalling through PI3Kgamma / Signaling by CSF1 (M-CSF) in myeloid cells /
endocytosis / G beta:gamma signalling through PI3Kgamma / Signaling by CSF1 (M-CSF) in myeloid cells /  kinase activity / positive regulation of cytosolic calcium ion concentration /
kinase activity / positive regulation of cytosolic calcium ion concentration /  angiogenesis /
angiogenesis /  adaptive immune response / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction /
adaptive immune response / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction /  non-specific serine/threonine protein kinase /
non-specific serine/threonine protein kinase /  protein kinase activity /
protein kinase activity /  immune response /
immune response /  inflammatory response / G protein-coupled receptor signaling pathway /
inflammatory response / G protein-coupled receptor signaling pathway /  phosphorylation / protein serine kinase activity /
phosphorylation / protein serine kinase activity /  innate immune response / protein serine/threonine kinase activity /
innate immune response / protein serine/threonine kinase activity /  ATP binding /
ATP binding /  membrane / identical protein binding /
membrane / identical protein binding /  plasma membrane /
plasma membrane /  cytosol /
cytosol /  cytoplasm
cytoplasm
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.55 Å
MOLECULAR REPLACEMENT / Resolution: 2.55 Å  Authors
Authors Canada, 1items
Canada, 1items  Citation
Citation Journal: Elife / Year: 2021
Journal: Elife / Year: 2021 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 7jwe.cif.gz
7jwe.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb7jwe.ent.gz
pdb7jwe.ent.gz PDB format
PDB format 7jwe.json.gz
7jwe.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/jw/7jwe
https://data.pdbj.org/pub/pdb/validation_reports/jw/7jwe ftp://data.pdbj.org/pub/pdb/validation_reports/jw/7jwe
ftp://data.pdbj.org/pub/pdb/validation_reports/jw/7jwe


 Links
Links Assembly
Assembly
 Components
Components
 Homo sapiens (human) / Gene: PIK3CG / Production host:
Homo sapiens (human) / Gene: PIK3CG / Production host: 
 Spodoptera frugiperda (fall armyworm)
Spodoptera frugiperda (fall armyworm) phosphatidylinositol-4,5-bisphosphate 3-kinase,
phosphatidylinositol-4,5-bisphosphate 3-kinase,  non-specific serine/threonine protein kinase
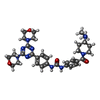
non-specific serine/threonine protein kinase Gedatolisib
Gedatolisib Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  CLSI
CLSI  / Beamline: 08ID-1 / Wavelength: 0.9795 Å
/ Beamline: 08ID-1 / Wavelength: 0.9795 Å : 0.9795 Å / Relative weight: 1
: 0.9795 Å / Relative weight: 1  Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller




















 PDBj
PDBj