+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7jhx | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of hEPG5 LIR/GABARAPL1 complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  SIGNALING PROTEIN / SIGNALING PROTEIN /  Complex Complex | ||||||
| Function / homology |  Function and homology information Function and homology informationnucleotide transport / cellular response to dsDNA / glycophagy / Tat protein binding /  GABA receptor binding / toll-like receptor 9 signaling pathway / cellular response to nitrogen starvation / GABA receptor binding / toll-like receptor 9 signaling pathway / cellular response to nitrogen starvation /  phosphatidylethanolamine binding / endocytic recycling / phosphatidylethanolamine binding / endocytic recycling /  Macroautophagy ...nucleotide transport / cellular response to dsDNA / glycophagy / Tat protein binding / Macroautophagy ...nucleotide transport / cellular response to dsDNA / glycophagy / Tat protein binding /  GABA receptor binding / toll-like receptor 9 signaling pathway / cellular response to nitrogen starvation / GABA receptor binding / toll-like receptor 9 signaling pathway / cellular response to nitrogen starvation /  phosphatidylethanolamine binding / endocytic recycling / phosphatidylethanolamine binding / endocytic recycling /  Macroautophagy / endosome to lysosome transport / Macroautophagy / endosome to lysosome transport /  beta-tubulin binding / autophagosome membrane / autophagosome maturation / beta-tubulin binding / autophagosome membrane / autophagosome maturation /  autophagosome assembly / autophagosome assembly /  autophagosome / cytoplasmic vesicle membrane / autophagosome / cytoplasmic vesicle membrane /  phospholipid binding / phospholipid binding /  autophagy / autophagy /  microtubule / microtubule /  lysosome / lysosome /  ubiquitin protein ligase binding / perinuclear region of cytoplasm / ubiquitin protein ligase binding / perinuclear region of cytoplasm /  Golgi apparatus / Golgi apparatus /  endoplasmic reticulum / endoplasmic reticulum /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.91 Å MOLECULAR REPLACEMENT / Resolution: 1.91 Å | ||||||
 Authors Authors | Cheung, Y.W.S. / Yip, C.K. | ||||||
| Funding support |  Canada, 1items Canada, 1items
| ||||||
 Citation Citation |  Journal: Commun Biol / Year: 2021 Journal: Commun Biol / Year: 2021Title: Insights on autophagosome-lysosome tethering from structural and biochemical characterization of human autophagy factor EPG5. Authors: Sung-Eun Nam / Yiu Wing Sunny Cheung / Thanh Ngoc Nguyen / Michael Gong / Samuel Chan / Michael Lazarou / Calvin K Yip /   Abstract: Pivotal to the maintenance of cellular homeostasis, macroautophagy (hereafter autophagy) is an evolutionarily conserved degradation system that involves sequestration of cytoplasmic material into the ...Pivotal to the maintenance of cellular homeostasis, macroautophagy (hereafter autophagy) is an evolutionarily conserved degradation system that involves sequestration of cytoplasmic material into the double-membrane autophagosome and targeting of this transport vesicle to the lysosome/late endosome for degradation. EPG5 is a large-sized metazoan protein proposed to serve as a tethering factor to enforce autophagosome-lysosome/late endosome fusion specificity, and its deficiency causes a severe multisystem disorder known as Vici syndrome. Here, we show that human EPG5 (hEPG5) adopts an extended "shepherd's staff" architecture. We find that hEPG5 binds preferentially to members of the GABARAP subfamily of human ATG8 proteins critical to autophagosome-lysosome fusion. The hEPG5-GABARAPs interaction, which is mediated by tandem LIR motifs that exhibit differential affinities, is required for hEPG5 recruitment to mitochondria during PINK1/Parkin-dependent mitophagy. Lastly, we find that the Vici syndrome mutation Gln336Arg does not affect the hEPG5's overall stability nor its ability to engage in interaction with the GABARAPs. Collectively, results from our studies reveal new insights into how hEPG5 recognizes mature autophagosome and establish a platform for examining the molecular effects of Vici syndrome disease mutations on hEPG5. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7jhx.cif.gz 7jhx.cif.gz | 126 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7jhx.ent.gz pdb7jhx.ent.gz | 97.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7jhx.json.gz 7jhx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jh/7jhx https://data.pdbj.org/pub/pdb/validation_reports/jh/7jhx ftp://data.pdbj.org/pub/pdb/validation_reports/jh/7jhx ftp://data.pdbj.org/pub/pdb/validation_reports/jh/7jhx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2r2qS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 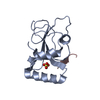
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 14479.526 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GABARAPL1, GEC1 / Production host: Homo sapiens (human) / Gene: GABARAPL1, GEC1 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9H0R8 Escherichia coli (E. coli) / References: UniProt: Q9H0R8#2: Protein/peptide | Mass: 1431.501 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)   Homo sapiens (human) / References: UniProt: Q9HCE0 Homo sapiens (human) / References: UniProt: Q9HCE0#3: Chemical |  Sulfate Sulfate#4: Water | ChemComp-HOH / |  Water WaterHas ligand of interest | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.43 Å3/Da / Density % sol: 49.37 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 8 Details: 0.2 M ammonium sulfate, 0.1 MES buffer pH 5.5, 29% (w/v) PEG 4000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1.00001 Å / Beamline: 5.0.2 / Wavelength: 1.00001 Å | |||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Mar 6, 2019 | |||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1.00001 Å / Relative weight: 1 : 1.00001 Å / Relative weight: 1 | |||||||||||||||||||||||||||
| Reflection | Resolution: 1.91→57 Å / Num. obs: 21346 / % possible obs: 93.4 % / Redundancy: 3.2 % / CC1/2: 0.985 / Rmerge(I) obs: 0.148 / Rpim(I) all: 0.097 / Rrim(I) all: 0.177 / Net I/σ(I): 4.6 / Num. measured all: 68435 / Scaling rejects: 52 | |||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Redundancy: 2.9 %
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2R2Q Resolution: 1.91→57 Å / SU ML: 0.22 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 24.86 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 77.14 Å2 / Biso mean: 25.4466 Å2 / Biso min: 8.26 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.91→57 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj


