+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7cby | ||||||
|---|---|---|---|---|---|---|---|
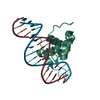
| Title | Structure of FOXG1 DNA binding domain bound to DBE2 DNA site | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TRANSCRIPTION / FOXG1 / TRANSCRIPTION / FOXG1 /  Forkhead box / Forkhead box /  transcription factors / FOXG1 syndrome / DBE2 transcription factors / FOXG1 syndrome / DBE2 | ||||||
| Function / homology |  Function and homology information Function and homology informationpyramidal neuron migration to cerebral cortex / : / FOXO-mediated transcription of cell cycle genes / neuron fate determination / dorsal/ventral pattern formation / axon midline choice point recognition / inner ear morphogenesis / positive regulation of neuroblast proliferation / Regulation of MECP2 expression and activity / negative regulation of neuron differentiation ...pyramidal neuron migration to cerebral cortex / : / FOXO-mediated transcription of cell cycle genes / neuron fate determination / dorsal/ventral pattern formation / axon midline choice point recognition / inner ear morphogenesis / positive regulation of neuroblast proliferation / Regulation of MECP2 expression and activity / negative regulation of neuron differentiation / positive regulation of cell cycle / regulation of mitotic cell cycle / positive regulation of neuron differentiation /  brain development / sequence-specific double-stranded DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / negative regulation of DNA-templated transcription / brain development / sequence-specific double-stranded DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / negative regulation of DNA-templated transcription /  chromatin / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / chromatin / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II /  DNA binding / DNA binding /  nucleoplasm / nucleoplasm /  nucleus nucleusSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.646 Å MOLECULAR REPLACEMENT / Resolution: 1.646 Å | ||||||
 Authors Authors | Dai, S.Y. / Li, J. / Chen, Y.H. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2020 Journal: J.Mol.Biol. / Year: 2020Title: Structural Basis for DNA Recognition by FOXG1 and the Characterization of Disease-causing FOXG1 Mutations. Authors: Dai, S. / Li, J. / Zhang, H. / Chen, X. / Guo, M. / Chen, Z. / Chen, Y. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7cby.cif.gz 7cby.cif.gz | 104.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7cby.ent.gz pdb7cby.ent.gz | 74.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7cby.json.gz 7cby.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cb/7cby https://data.pdbj.org/pub/pdb/validation_reports/cb/7cby ftp://data.pdbj.org/pub/pdb/validation_reports/cb/7cby ftp://data.pdbj.org/pub/pdb/validation_reports/cb/7cby | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6akoS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: DNA chain | Mass: 4860.159 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: SF file contains Friedel pairs. / Source: (synth.)   Homo sapiens (human) Homo sapiens (human) |
|---|---|
| #2: DNA chain | Mass: 4932.272 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: SF file contains Friedel pairs. / Source: (synth.)   Homo sapiens (human) Homo sapiens (human) |
| #3: Protein |  FOX proteins / Brain factor 1 / BF1 / Brain factor 2 / hBF-2 / Forkhead box protein G1A / Forkhead box protein G1B ...Brain factor 1 / BF1 / Brain factor 2 / hBF-2 / Forkhead box protein G1A / Forkhead box protein G1B / Forkhead box protein G1C / Forkhead-related protein FKHL1 / HFK1 / Forkhead-related protein FKHL2 / HFK2 / Forkhead-related protein FKHL3 / HFK3 FOX proteins / Brain factor 1 / BF1 / Brain factor 2 / hBF-2 / Forkhead box protein G1A / Forkhead box protein G1B ...Brain factor 1 / BF1 / Brain factor 2 / hBF-2 / Forkhead box protein G1A / Forkhead box protein G1B / Forkhead box protein G1C / Forkhead-related protein FKHL1 / HFK1 / Forkhead-related protein FKHL2 / HFK2 / Forkhead-related protein FKHL3 / HFK3Mass: 12485.281 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: SF file contains Friedel pairs. / Source: (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Gene: FOXG1, FKH2, FKHL1, FKHL2, FKHL3, FKHL4, FOXG1A, FOXG1B, FOXG1C Production host:   Escherichia coli O103:H2 str. 12009 (bacteria) Escherichia coli O103:H2 str. 12009 (bacteria)References: UniProt: P55316 |
| #4: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol |
| #5: Water | ChemComp-HOH /  Water Water |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.44 Å3/Da / Density % sol: 49.64 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop Details: 80 mM NaCl, 40 mM Sodium cacodylate, pH 6.0, 45% 2-Methyl-2,4-pentanediol, 12 mM spermine |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL17U1 / Wavelength: 0.979 Å / Beamline: BL17U1 / Wavelength: 0.979 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: May 17, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.979 Å / Relative weight: 1 : 0.979 Å / Relative weight: 1 |
| Reflection | Resolution: 1.64→22.9 Å / Num. obs: 26897 / % possible obs: 98.9 % / Redundancy: 13.7 % / Rrim(I) all: 0.035 / Net I/σ(I): 20.7 |
| Reflection shell | Resolution: 1.64→1.7 Å / Num. unique obs: 2635 / Rpim(I) all: 0.312 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6AKO Resolution: 1.646→22.871 Å / SU ML: 0.12 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 16.2 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 78.4 Å2 / Biso mean: 21.948 Å2 / Biso min: 4.27 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.646→22.871 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj













































