[English] 日本語
 Yorodumi
Yorodumi- PDB-6yi1: Crystal structure of human glutaminyl cyclase in complex with Glu... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6yi1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human glutaminyl cyclase in complex with Glu(gamma-hydrazide)-Phe-Ala | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  Alpha-Beta Protein / Alpha-Beta Protein /  Metalloprotein / Intermediate Metalloprotein / Intermediate | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpeptidyl-pyroglutamic acid biosynthetic process, using glutaminyl-peptide cyclotransferase /  glutaminyl-peptide cyclotransferase / glutaminyl-peptide cyclotransferase /  glutaminyl-peptide cyclotransferase activity / protein modification process / specific granule lumen / tertiary granule lumen / ficolin-1-rich granule lumen / Neutrophil degranulation / extracellular exosome / zinc ion binding / extracellular region glutaminyl-peptide cyclotransferase activity / protein modification process / specific granule lumen / tertiary granule lumen / ficolin-1-rich granule lumen / Neutrophil degranulation / extracellular exosome / zinc ion binding / extracellular regionSimilarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)Synthetic construct (others) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.92 Å MOLECULAR REPLACEMENT / Resolution: 1.92 Å | ||||||||||||
 Authors Authors | Kupski, O. / Sautner, V. / Tittmann, K. | ||||||||||||
| Funding support |  Germany, 3items Germany, 3items
| ||||||||||||
 Citation Citation |  Journal: Biochemistry / Year: 2020 Journal: Biochemistry / Year: 2020Title: Hydrazides Are Potent Transition-State Analogues for Glutaminyl Cyclase Implicated in the Pathogenesis of Alzheimer's Disease. Authors: Kupski, O. / Funk, L.M. / Sautner, V. / Seifert, F. / Worbs, B. / Ramsbeck, D. / Meyer, F. / Diederichsen, U. / Buchholz, M. / Schilling, S. / Demuth, H.U. / Tittmann, K. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6yi1.cif.gz 6yi1.cif.gz | 309 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6yi1.ent.gz pdb6yi1.ent.gz | 246.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6yi1.json.gz 6yi1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yi/6yi1 https://data.pdbj.org/pub/pdb/validation_reports/yi/6yi1 ftp://data.pdbj.org/pub/pdb/validation_reports/yi/6yi1 ftp://data.pdbj.org/pub/pdb/validation_reports/yi/6yi1 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6yjyC  2afmS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 5 molecules ABCDE
| #1: Protein |  / Glutaminyl cyclase / sQC / Glutaminyl-tRNA cyclotransferase / Glutamyl cyclase / EC / Glutaminyl cyclase / sQC / Glutaminyl-tRNA cyclotransferase / Glutamyl cyclase / ECMass: 37553.410 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: QPCT / Production host: Homo sapiens (human) / Gene: QPCT / Production host:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria)References: UniProt: Q16769,  glutaminyl-peptide cyclotransferase glutaminyl-peptide cyclotransferase#2: Protein/peptide | Mass: 361.419 Da / Num. of mol.: 3 / Source method: obtained synthetically / Source: (synth.) Synthetic construct (others) |
|---|
-Non-polymers , 9 types, 462 molecules 


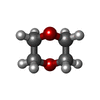













| #3: Chemical | ChemComp-SO4 /  Sulfate Sulfate#4: Chemical | ChemComp-ZN / #5: Chemical | ChemComp-GOL /  Glycerol Glycerol#6: Chemical | ChemComp-DIO / |  1,4-Dioxane 1,4-Dioxane#7: Chemical |  Polyethylene glycol Polyethylene glycol#8: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#9: Chemical |  Polyethylene glycol Polyethylene glycol#10: Chemical |  MES (buffer) MES (buffer)#11: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.08 Å3/Da / Density % sol: 60.08 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop Details: 1.6M ammonium sulfate, 0.1M MES pH 6.5, 4% (v/v) 1,4-Dioxane |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  PETRA III, EMBL c/o DESY PETRA III, EMBL c/o DESY  / Beamline: P13 (MX1) / Wavelength: 0.85 Å / Beamline: P13 (MX1) / Wavelength: 0.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Nov 8, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 0.85 Å / Relative weight: 1 : 0.85 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.92→88.279 Å / Num. obs: 70565 / % possible obs: 99.4 % / Redundancy: 5.933 % / Biso Wilson estimate: 26.39 Å2 / CC1/2: 0.997 / Rmerge(I) obs: 0.119 / Rrim(I) all: 0.129 / Χ2: 0.957 / Net I/σ(I): 9.89 / Num. measured all: 418679 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2AFM Resolution: 1.92→88.279 Å / SU ML: 0.2 / Cross valid method: THROUGHOUT / σ(F): 1.36 / Phase error: 17.73
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 1.1 Å / VDW probe radii: 1.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 121.67 Å2 / Biso mean: 30.3477 Å2 / Biso min: 5.58 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.92→88.279 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







