+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6vxg | ||||||
|---|---|---|---|---|---|---|---|
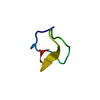
| Title | Structure of the C-terminal Domain of RAGE and Its Inhibitor | ||||||
 Components Components | Advanced glycosylation end product-specific receptor | ||||||
 Keywords Keywords |  SIGNALING PROTEIN/INHIBITOR / Inhibitor / SIGNALING PROTEIN/INHIBITOR / Inhibitor /  Complex / Complex /  Diabetes / Receptor for Advanced Glycated End Products / Diabetes / Receptor for Advanced Glycated End Products /  Inflammation / Inflammation /  SIGNALING PROTEIN / SIGNALING PROTEIN /  SIGNALING PROTEIN-INHIBITOR complex SIGNALING PROTEIN-INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of CD4-positive, alpha-beta T cell activation / advanced glycation end-product receptor activity / negative regulation of blood circulation / regulation of T cell mediated cytotoxicity / positive regulation of endothelin production / glucose mediated signaling pathway / negative regulation of long-term synaptic depression / positive regulation of monocyte extravasation / positive regulation of aspartic-type endopeptidase activity involved in amyloid precursor protein catabolic process / positive regulation of dendritic cell differentiation ...regulation of CD4-positive, alpha-beta T cell activation / advanced glycation end-product receptor activity / negative regulation of blood circulation / regulation of T cell mediated cytotoxicity / positive regulation of endothelin production / glucose mediated signaling pathway / negative regulation of long-term synaptic depression / positive regulation of monocyte extravasation / positive regulation of aspartic-type endopeptidase activity involved in amyloid precursor protein catabolic process / positive regulation of dendritic cell differentiation / regulation of p38MAPK cascade / regulation of non-canonical NF-kappaB signal transduction / scavenger receptor activity / induction of positive chemotaxis /  transcytosis / laminin receptor activity / protein localization to membrane / positive regulation of monocyte chemotactic protein-1 production / positive regulation of heterotypic cell-cell adhesion / S100 protein binding / regulation of long-term synaptic potentiation / regulation of spontaneous synaptic transmission / positive regulation of p38MAPK cascade / negative regulation of interleukin-10 production / positive regulation of activated T cell proliferation / response to amyloid-beta / TRAF6 mediated NF-kB activation / Advanced glycosylation endproduct receptor signaling / transport across blood-brain barrier / negative regulation of long-term synaptic potentiation / positive regulation of chemokine production / positive regulation of interleukin-12 production / positive regulation of interleukin-1 beta production / astrocyte activation / positive regulation of JNK cascade / microglial cell activation / TAK1-dependent IKK and NF-kappa-B activation / transcytosis / laminin receptor activity / protein localization to membrane / positive regulation of monocyte chemotactic protein-1 production / positive regulation of heterotypic cell-cell adhesion / S100 protein binding / regulation of long-term synaptic potentiation / regulation of spontaneous synaptic transmission / positive regulation of p38MAPK cascade / negative regulation of interleukin-10 production / positive regulation of activated T cell proliferation / response to amyloid-beta / TRAF6 mediated NF-kB activation / Advanced glycosylation endproduct receptor signaling / transport across blood-brain barrier / negative regulation of long-term synaptic potentiation / positive regulation of chemokine production / positive regulation of interleukin-12 production / positive regulation of interleukin-1 beta production / astrocyte activation / positive regulation of JNK cascade / microglial cell activation / TAK1-dependent IKK and NF-kappa-B activation /  regulation of synaptic plasticity / regulation of synaptic plasticity /  fibrillar center / response to wounding / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to amyloid-beta / positive regulation of interleukin-6 production / transmembrane signaling receptor activity / neuron projection development / positive regulation of tumor necrosis factor production / fibrillar center / response to wounding / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to amyloid-beta / positive regulation of interleukin-6 production / transmembrane signaling receptor activity / neuron projection development / positive regulation of tumor necrosis factor production /  cell junction / cell junction /  signaling receptor activity / positive regulation of NF-kappaB transcription factor activity / signaling receptor activity / positive regulation of NF-kappaB transcription factor activity /  amyloid-beta binding / amyloid-beta binding /  regulation of inflammatory response / postsynapse / molecular adaptor activity / learning or memory / cell surface receptor signaling pathway / positive regulation of ERK1 and ERK2 cascade / response to hypoxia / regulation of inflammatory response / postsynapse / molecular adaptor activity / learning or memory / cell surface receptor signaling pathway / positive regulation of ERK1 and ERK2 cascade / response to hypoxia /  inflammatory response / apical plasma membrane / positive regulation of protein phosphorylation / protein-containing complex binding / inflammatory response / apical plasma membrane / positive regulation of protein phosphorylation / protein-containing complex binding /  cell surface / extracellular region / identical protein binding / cell surface / extracellular region / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  SOLUTION NMR / SOLUTION NMR /  simulated annealing simulated annealing | ||||||
 Authors Authors | Ramirez, L. / Shekhtman, A. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Sci Transl Med / Year: 2021 Journal: Sci Transl Med / Year: 2021Title: Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Authors: Manigrasso, M.B. / Rabbani, P. / Egana-Gorrono, L. / Quadri, N. / Frye, L. / Zhou, B. / Reverdatto, S. / Ramirez, L.S. / Dansereau, S. / Pan, J. / Li, H. / D'Agati, V.D. / Ramasamy, R. / ...Authors: Manigrasso, M.B. / Rabbani, P. / Egana-Gorrono, L. / Quadri, N. / Frye, L. / Zhou, B. / Reverdatto, S. / Ramirez, L.S. / Dansereau, S. / Pan, J. / Li, H. / D'Agati, V.D. / Ramasamy, R. / DeVita, R.J. / Shekhtman, A. / Schmidt, A.M. #1:  Journal: J. Biol. Chem. / Year: 2012 Journal: J. Biol. Chem. / Year: 2012Title: Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. Authors: Rai, V. / Maldonado, A.Y. / Burz, D.S. / Reverdatto, S. / Yan, S.F. / Schmidt, A.M. / Shekhtman, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6vxg.cif.gz 6vxg.cif.gz | 144.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6vxg.ent.gz pdb6vxg.ent.gz | 103.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6vxg.json.gz 6vxg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vx/6vxg https://data.pdbj.org/pub/pdb/validation_reports/vx/6vxg ftp://data.pdbj.org/pub/pdb/validation_reports/vx/6vxg ftp://data.pdbj.org/pub/pdb/validation_reports/vx/6vxg | HTTPS FTP |
|---|
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data | |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 5035.241 Da / Num. of mol.: 1 / Fragment: C-terminal residues 363-404 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: AGER, RAGE / Production host: Homo sapiens (human) / Gene: AGER, RAGE / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: Q15109 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: Q15109 |
|---|---|
| #2: Chemical | ChemComp-RQV / |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Type: solution Contents: 300 uM [U-100% 13C; U-100% 15N] C-terminal RAGE, 90% H2O/10% D2O Details: Recombinant protein bound to small molecule ligand (N-(4-(7-cyano-4-(morpholin-4-ylmethyl)quinoline-2-yl)phenyl)acetamide) Label: 13C_15N_sample / Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample | Conc.: 300 uM / Component: C-terminal RAGE / Isotopic labeling: [U-100% 13C; U-100% 15N] |
| Sample conditions | Details: 10 mM potassium phosphate buffer, pH 7.0 / Ionic strength: 10 mM / Label: condition_1 / pH: 7 / Pressure: 1 atm / Temperature: 298 K |
-NMR measurement
| NMR spectrometer | Type: Bruker AVANCE / Manufacturer: Bruker / Model : AVANCE / Field strength: 600 MHz / Details: QCI HCN cryoprobe : AVANCE / Field strength: 600 MHz / Details: QCI HCN cryoprobe |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method:  simulated annealing / Software ordinal: 1 simulated annealing / Software ordinal: 1 | ||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the least restraint violations Conformers calculated total number: 200 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj














