[English] 日本語
 Yorodumi
Yorodumi- PDB-6uzw: Crystal structure of GLUN1/GLUN2A ligand-binding domain in comple... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6uzw | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of GLUN1/GLUN2A ligand-binding domain in complex with glycine and UBP791 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  NMDAR / ligand-binding domain / NMDAR / ligand-binding domain /  antagonist antagonist | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to ammonium ion / directional locomotion / pons maturation /  regulation of cell communication / positive regulation of Schwann cell migration / EPHB-mediated forward signaling / serotonin metabolic process / Assembly and cell surface presentation of NMDA receptors / olfactory learning / regulation of cell communication / positive regulation of Schwann cell migration / EPHB-mediated forward signaling / serotonin metabolic process / Assembly and cell surface presentation of NMDA receptors / olfactory learning /  conditioned taste aversion ...response to ammonium ion / directional locomotion / pons maturation / conditioned taste aversion ...response to ammonium ion / directional locomotion / pons maturation /  regulation of cell communication / positive regulation of Schwann cell migration / EPHB-mediated forward signaling / serotonin metabolic process / Assembly and cell surface presentation of NMDA receptors / olfactory learning / regulation of cell communication / positive regulation of Schwann cell migration / EPHB-mediated forward signaling / serotonin metabolic process / Assembly and cell surface presentation of NMDA receptors / olfactory learning /  conditioned taste aversion / protein localization to postsynaptic membrane / conditioned taste aversion / protein localization to postsynaptic membrane /  dendritic branch / regulation of respiratory gaseous exchange / propylene metabolic process / response to glycine / response to other organism / dendritic branch / regulation of respiratory gaseous exchange / propylene metabolic process / response to glycine / response to other organism /  sleep / cellular response to magnesium ion / response to methylmercury / voltage-gated monoatomic cation channel activity / locomotion / response to morphine / glutamate-gated calcium ion channel activity / cellular response to dsRNA / response to carbohydrate / regulation of monoatomic cation transmembrane transport / dendritic spine organization / cellular response to lipid / sleep / cellular response to magnesium ion / response to methylmercury / voltage-gated monoatomic cation channel activity / locomotion / response to morphine / glutamate-gated calcium ion channel activity / cellular response to dsRNA / response to carbohydrate / regulation of monoatomic cation transmembrane transport / dendritic spine organization / cellular response to lipid /  NMDA glutamate receptor activity / NMDA selective glutamate receptor complex / RAF/MAP kinase cascade / Synaptic adhesion-like molecules / parallel fiber to Purkinje cell synapse / calcium ion transmembrane import into cytosol / response to manganese ion / protein heterotetramerization / NMDA glutamate receptor activity / NMDA selective glutamate receptor complex / RAF/MAP kinase cascade / Synaptic adhesion-like molecules / parallel fiber to Purkinje cell synapse / calcium ion transmembrane import into cytosol / response to manganese ion / protein heterotetramerization /  glutamate binding / positive regulation of reactive oxygen species biosynthetic process / cellular response to zinc ion / neuromuscular process / glutamate binding / positive regulation of reactive oxygen species biosynthetic process / cellular response to zinc ion / neuromuscular process /  regulation of synapse assembly / regulation of synapse assembly /  action potential / action potential /  glycine binding / positive regulation of calcium ion transport into cytosol / male mating behavior / regulation of dendrite morphogenesis / glycine binding / positive regulation of calcium ion transport into cytosol / male mating behavior / regulation of dendrite morphogenesis /  regulation of axonogenesis / dopamine metabolic process / spinal cord development / suckling behavior / regulation of axonogenesis / dopamine metabolic process / spinal cord development / suckling behavior /  startle response / regulation of neuronal synaptic plasticity / response to amine / monoatomic cation transmembrane transport / startle response / regulation of neuronal synaptic plasticity / response to amine / monoatomic cation transmembrane transport /  regulation of NMDA receptor activity / regulation of NMDA receptor activity /  social behavior / social behavior /  associative learning / positive regulation of excitatory postsynaptic potential / monoatomic cation transport / ligand-gated monoatomic ion channel activity / response to light stimulus / associative learning / positive regulation of excitatory postsynaptic potential / monoatomic cation transport / ligand-gated monoatomic ion channel activity / response to light stimulus /  excitatory synapse / Unblocking of NMDA receptors, glutamate binding and activation / neuron development / positive regulation of dendritic spine maintenance / excitatory synapse / Unblocking of NMDA receptors, glutamate binding and activation / neuron development / positive regulation of dendritic spine maintenance /  glutamate receptor binding / regulation of postsynaptic membrane potential / glutamate receptor binding / regulation of postsynaptic membrane potential /  phosphatase binding / calcium ion homeostasis / cellular response to manganese ion / phosphatase binding / calcium ion homeostasis / cellular response to manganese ion /  prepulse inhibition / prepulse inhibition /  long-term memory / regulation of neuron apoptotic process / glutamate-gated receptor activity / long-term memory / regulation of neuron apoptotic process / glutamate-gated receptor activity /  synaptic cleft / presynaptic active zone membrane / response to fungicide / monoatomic cation channel activity / sensory perception of pain / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / response to amphetamine / synaptic cleft / presynaptic active zone membrane / response to fungicide / monoatomic cation channel activity / sensory perception of pain / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / response to amphetamine /  excitatory postsynaptic potential / hippocampal mossy fiber to CA3 synapse / excitatory postsynaptic potential / hippocampal mossy fiber to CA3 synapse /  regulation of membrane potential / ionotropic glutamate receptor signaling pathway / regulation of membrane potential / ionotropic glutamate receptor signaling pathway /  cell adhesion molecule binding / cell adhesion molecule binding /  neurogenesis / positive regulation of synaptic transmission, glutamatergic / adult locomotory behavior / response to cocaine / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / neurogenesis / positive regulation of synaptic transmission, glutamatergic / adult locomotory behavior / response to cocaine / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential /  synaptic membrane / synaptic membrane /  synaptic transmission, glutamatergic / synaptic transmission, glutamatergic /  learning / long-term synaptic potentiation / hippocampus development / cellular response to amino acid stimulus / postsynaptic density membrane / regulation of long-term neuronal synaptic plasticity learning / long-term synaptic potentiation / hippocampus development / cellular response to amino acid stimulus / postsynaptic density membrane / regulation of long-term neuronal synaptic plasticitySimilarity search - Function | |||||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.13 Å MOLECULAR REPLACEMENT / Resolution: 2.13 Å | |||||||||
 Authors Authors | Wang, J.X. / Furukawa, H. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structural basis of subtype-selective competitive antagonism for GluN2C/2D-containing NMDA receptors. Authors: Wang, J.X. / Irvine, M.W. / Burnell, E.S. / Sapkota, K. / Thatcher, R.J. / Li, M. / Simorowski, N. / Volianskis, A. / Collingridge, G.L. / Monaghan, D.T. / Jane, D.E. / Furukawa, H. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6uzw.cif.gz 6uzw.cif.gz | 288 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6uzw.ent.gz pdb6uzw.ent.gz | 192.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6uzw.json.gz 6uzw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uz/6uzw https://data.pdbj.org/pub/pdb/validation_reports/uz/6uzw ftp://data.pdbj.org/pub/pdb/validation_reports/uz/6uzw ftp://data.pdbj.org/pub/pdb/validation_reports/uz/6uzw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6uz6C  6uzgC  6uzrC  6uzxC  4nf6S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 33340.031 Da / Num. of mol.: 1 Fragment: ligand-binding domain (UNP residues 415-565,684-821) Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Grin1, Nmdar1 / Production host: Rattus norvegicus (Norway rat) / Gene: Grin1, Nmdar1 / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): OrigamiB (DE3) / References: UniProt: P35439 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): OrigamiB (DE3) / References: UniProt: P35439 |
|---|---|
| #2: Protein | Mass: 31698.221 Da / Num. of mol.: 1 Fragment: ligand-binding domain (UNP residues 402-539,661-802) Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Grin2a / Production host: Rattus norvegicus (Norway rat) / Gene: Grin2a / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): OrigamiB (DE3) / References: UniProt: Q00959 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): OrigamiB (DE3) / References: UniProt: Q00959 |
| #3: Chemical | ChemComp-GLY /  Glycine Glycine |
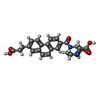
| #4: Chemical | ChemComp-QM4 / ( |
| #5: Water | ChemComp-HOH /  Water Water |
| Has ligand of interest | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.35 Å3/Da / Density % sol: 47.57 % |
|---|---|
Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop / pH: 7 Details: 100 mM HEPES, pH 7.0, 75 mM sodium chloride, 18% PEG2000 MME |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS-II NSLS-II  / Beamline: 17-ID-1 / Wavelength: 0.92013 Å / Beamline: 17-ID-1 / Wavelength: 0.92013 Å |
| Detector | Type: DECTRIS EIGER X 9M / Detector: PIXEL / Date: Feb 9, 2018 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.92013 Å / Relative weight: 1 : 0.92013 Å / Relative weight: 1 |
| Reflection | Resolution: 2.13→69.74 Å / Num. obs: 26485 / % possible obs: 99.9 % / Redundancy: 6.6 % / Biso Wilson estimate: 46.43 Å2 / Rmerge(I) obs: 0.104 / Net I/σ(I): 12.7 |
| Reflection shell | Resolution: 2.52→2.56 Å / Rmerge(I) obs: 0.903 / Num. unique obs: 178 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 4NF6 Resolution: 2.13→69.74 Å / SU ML: 0.2719 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 32.7206 Details: Note: we used STARANISO; overall statistics as shown on collection statistics refer to data to 2.52 A resolution, but some anisotropic data went to 2.13 A which were included in the refinement
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 53.37 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.13→69.74 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 15.3556806266 Å / Origin y: 14.7895537039 Å / Origin z: -35.5446108033 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller












 PDBj
PDBj