[English] 日本語
 Yorodumi
Yorodumi- PDB-6tez: Crystal Structure of full-length Human Lysyl Hydroxylase LH3 - Va... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6tez | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of full-length Human Lysyl Hydroxylase LH3 - Val80Lys mutant - Cocrystal with Fe2+, Mn2+, UDP-Glucuronic Acid | |||||||||||||||
 Components Components | Multifunctional procollagen lysine hydroxylase and glycosyltransferase LH3 | |||||||||||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  Collagen / Collagen /  lysyl hydroxylase / lysyl hydroxylase /  hydroxylysine / hydroxylysine /  galactosyltransferase / galactosyltransferase /  glucosyltransferase glucosyltransferase | |||||||||||||||
| Function / homology |  Function and homology information Function and homology information procollagen galactosyltransferase / procollagen galactosyltransferase /  procollagen glucosyltransferase / peptidyl-lysine hydroxylation / procollagen glucosyltransferase / peptidyl-lysine hydroxylation /  procollagen glucosyltransferase activity / hydroxylysine biosynthetic process / procollagen glucosyltransferase activity / hydroxylysine biosynthetic process /  procollagen galactosyltransferase activity / procollagen galactosyltransferase activity /  procollagen-lysine 5-dioxygenase / procollagen-lysine 5-dioxygenase /  procollagen-lysine 5-dioxygenase activity / procollagen-lysine 5-dioxygenase activity /  basement membrane assembly / epidermis morphogenesis ... basement membrane assembly / epidermis morphogenesis ... procollagen galactosyltransferase / procollagen galactosyltransferase /  procollagen glucosyltransferase / peptidyl-lysine hydroxylation / procollagen glucosyltransferase / peptidyl-lysine hydroxylation /  procollagen glucosyltransferase activity / hydroxylysine biosynthetic process / procollagen glucosyltransferase activity / hydroxylysine biosynthetic process /  procollagen galactosyltransferase activity / procollagen galactosyltransferase activity /  procollagen-lysine 5-dioxygenase / procollagen-lysine 5-dioxygenase /  procollagen-lysine 5-dioxygenase activity / procollagen-lysine 5-dioxygenase activity /  basement membrane assembly / epidermis morphogenesis / Collagen biosynthesis and modifying enzymes / collagen metabolic process / endothelial cell morphogenesis / protein O-linked glycosylation / basement membrane assembly / epidermis morphogenesis / Collagen biosynthesis and modifying enzymes / collagen metabolic process / endothelial cell morphogenesis / protein O-linked glycosylation /  L-ascorbic acid binding / collagen fibril organization / neural tube development / lung morphogenesis / L-ascorbic acid binding / collagen fibril organization / neural tube development / lung morphogenesis /  small molecule binding / small molecule binding /  rough endoplasmic reticulum / rough endoplasmic reticulum /  trans-Golgi network / trans-Golgi network /  protein localization / protein localization /  vasodilation / collagen-containing extracellular matrix / in utero embryonic development / iron ion binding / vasodilation / collagen-containing extracellular matrix / in utero embryonic development / iron ion binding /  endoplasmic reticulum lumen / endoplasmic reticulum membrane / endoplasmic reticulum lumen / endoplasmic reticulum membrane /  Golgi apparatus / Golgi apparatus /  endoplasmic reticulum / endoplasmic reticulum /  extracellular space / extracellular exosome / extracellular space / extracellular exosome /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å MOLECULAR REPLACEMENT / Resolution: 2.7 Å | |||||||||||||||
 Authors Authors | Chiapparino, A. / De Giorgi, F. / Scietti, L. / Faravelli, S. / Roscioli, T. / Forneris, F. | |||||||||||||||
| Funding support |  Italy, 4items Italy, 4items
| |||||||||||||||
 Citation Citation |  Journal: Int J Mol Sci / Year: 2023 Journal: Int J Mol Sci / Year: 2023Title: Identification of Regulatory Molecular 'Hot Spots' for LH/PLOD Collagen Glycosyltransferase Activity Authors: Mattoteia, D. / Chiapparino, A. / Fumagalli, M. / De Marco, M. / De Giorgi, F. / Negro, L. / Pinnola, A. / Faravelli, S. / Roscioli, T. / Scietti, L. / Forneris, F. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6tez.cif.gz 6tez.cif.gz | 362.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6tez.ent.gz pdb6tez.ent.gz | 244.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6tez.json.gz 6tez.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/te/6tez https://data.pdbj.org/pub/pdb/validation_reports/te/6tez ftp://data.pdbj.org/pub/pdb/validation_reports/te/6tez ftp://data.pdbj.org/pub/pdb/validation_reports/te/6tez | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6te3C  6tecC  6tesC  6teuC  6texC  6fxkS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 82814.461 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Val80Lys Mutation - First Ser residue and last three Ala residues were introduced by molecular cloning Source: (gene. exp.)   Homo sapiens (human) / Gene: PLOD3 / Cell line (production host): HEK293F / Production host: Homo sapiens (human) / Gene: PLOD3 / Cell line (production host): HEK293F / Production host:   Homo sapiens (human) Homo sapiens (human)References: UniProt: O60568,  procollagen-lysine 5-dioxygenase, procollagen-lysine 5-dioxygenase,  procollagen galactosyltransferase, procollagen galactosyltransferase,  procollagen glucosyltransferase procollagen glucosyltransferase |
|---|
-Sugars , 2 types, 2 molecules 
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / Mass: 424.401 Da / Num. of mol.: 1 / Mass: 424.401 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
|---|---|
| #8: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine |
-Non-polymers , 6 types, 80 molecules 

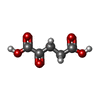








| #3: Chemical | ChemComp-UGA /  Uridine diphosphate glucuronic acid Uridine diphosphate glucuronic acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #4: Chemical |  Glycerol Glycerol#5: Chemical | ChemComp-AKG / |  Α-Ketoglutaric acid Α-Ketoglutaric acid#6: Chemical | #7: Chemical | ChemComp-MN / | #9: Water | ChemComp-HOH / |  Water Water |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.31 Å3/Da / Density % sol: 62.87 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.8 Details: 600 mM sodium formate, 12% PGA-LM, 100 mM HEPES/NaOH, 500 uM FeCl2, 500 uM MnCl2, 1 mM UDP-Glucuronic Acid Temp details: cold room |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 1 Å / Beamline: X06SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Nov 28, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.7→49 Å / Num. obs: 30477 / % possible obs: 99.5 % / Redundancy: 6.5 % / Biso Wilson estimate: 41.43 Å2 / CC1/2: 0.996 / Rmerge(I) obs: 0.138 / Rpim(I) all: 0.088 / Net I/σ(I): 9.4 |
| Reflection shell | Resolution: 2.7→2.83 Å / Rmerge(I) obs: 1.312 / Mean I/σ(I) obs: 1.3 / Num. unique obs: 3911 / CC1/2: 0.58 / Rpim(I) all: 0.902 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 6FXK Resolution: 2.7→49 Å / SU ML: 0.3007 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 23.2911
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 43.73 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.7→49 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj














